CELL MEMBRANES-STRUCTURE AND TRANSPORT
MEMBRANE TRANSPORT
One of the primary functions of the membrane is to separate the intracellular environment from its extracellular environment. This separation is crucial for the maintenance of the proper conditions for cell function. In order to perform this important function, the membrane must regulate what enters and leaves the cell. For example, the proper nutrients must be allowed to enter, and wastes must be allowed to leave the cell. Additionally, some things must not be permitted entrance to or exit from the cell. In this section we will discuss the ways that various substances are moved across the plasma membrane.
Passive vs Active Processes
Processes that move substances across membranes can be grouped into two general categories based on whether the process requires an input of cellular energy or not. If no energy input is required for the transport, then we say particles move via a passive transport process. On the other hand, if the process requires cellular energy, usually in the form of ATP, then it is an active transport process.
Simple Diffusion
Diffusion is a process that results from the fact that molecules are constantly in a state of random movement. They move in a straight line until they collide with another molecule and then move off in a different direction. If there is an initial, unequal distribution of the molecules (i.e. more concentrated in one area than another) the constant random movement and collisions cause them to eventually become equally distributed. This process of gradual movement from where they are more concentrated to where they are less concentrated is called diffusion. We refer to the concentration difference as the concentration gradient.
Therefore, substances diffuse down their concentration gradients (from high to low concentration). Once the molecules are evenly distributed, we say that we have reached a state of diffusion equilibrium and even though the molecules are still moving, there is no longer any net change in concentration. You can observe this phenomenon by carefully placing a drop of food coloring into a glass of water. The dye gradually moves through the liquid until it is evenly dispersed in the water. In the body, if the material in question can pass through the cell membrane without the aid of a membrane protein, we refer to the process as simple diffusion. Solutes that cross the membrane by simple diffusion tend to be hydrophobic. Examples of substance that cross the membrane by simple diffusion are the gasses CO2 and O2. The following video demonstrates the process of diffusion and explores some of the factors that influence the rate of diffusion. See if you can answer the questions posed in the video before reading the next section.
http://www.youtube.com/watch?v=H7QsDs8ZRMI (Transcription Available)
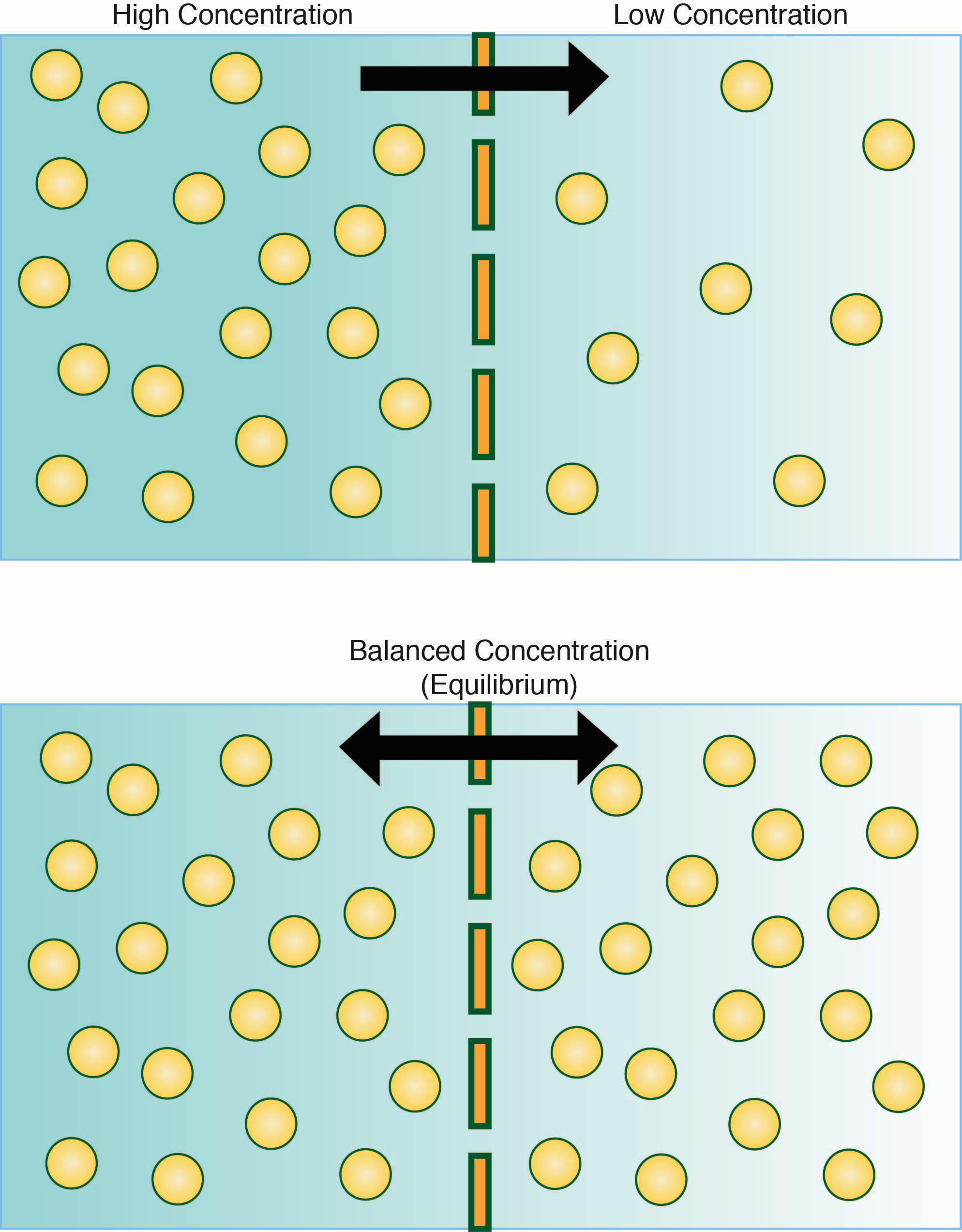
Image created by BYU-I student, Hannah Crowder, 2013 .
(Top panel) Diffusion of solute from right (high concentration) to the left (low concentration) until an equilibrium is established. Once a diffusion equilibrium exists, there will no longer be any net movements of solute (lower panel).
Factors That Affect the Rate of Diffusion
The rate at which the solute diffuses is affected by several factors.
Concentration gradient: The greater the difference between the concentrations on the two sides of the membrane, the faster the rate of diffusion.
Temperature: The higher the temperature, the faster molecules move. So, as temperature increases, the rate of diffusion increases.
Size of molecule: Smaller molecules tend to travel further before colliding with other molecules, so diffusion rates are faster for smaller molecules.
Viscosity of the medium: The viscosity is a measure of the "thickness" of the solvent. An increase in viscosity decreases the rate of diffusion.
Membrane permeability: Since we are talking about movement of solutes into and out of the cell, the permeability of the membrane to the solute will affect how fast solutes diffuse across the cell membrane. For example, ions and other charged molecules that are hydrophilic do not readily cross the membrane due to the hydrophobic core of the bilayer. Conversely, oxygen and carbon dioxide, both non-polar molecules, can readily diffuse through the membrane.
Surface area: The greater the surface area of the membrane, the faster the rate of diffusion across the membrane. Areas in our bodies, where diffusion is especially important, typically employ structural modifications that increase the available surface area. For example, in the lungs, the hundreds of millions of small alveoli have a total surface area of nearly 70 square meters for gas exchange! Approximately the same size as a typical 2 bedroom apartment in Rexburg.
Distance: Diffusion is quite rapid over short distances but gets slower the further it goes. The time it takes for something to diffuse is proportional to the square of the distance. Therefore, if it takes 1 second to diffuse 1 cm, it would take 100 seconds to diffuse 10 cm, and 10,000 seconds to diffuse 100 cm. So to go 100 times further takes 10,000 times longer. In the body, diffusion is quite sufficient to cross the thin cell membrane, but to travel long distances by diffusion would be very slow. This is why we have other mechanisms like the blood circulation for moving substances long distances.
Facilitated diffusion
Not all solutes can pass directly through cell membranes. Molecules that are large, or that have an electrical charge, generally are prevented from moving through the membrane. However, many of these solutes need be able to enter or leave the cell. So how does the cell solve this dilemma? Recall that embedded in the cell membrane are several types of proteins that pass completely through the membrane, the integral membrane proteins. There are a number of specialized integral proteins what assist in the diffusion of solutes across the membrane. This type of diffusion is referred to as facilitated diffusion. Facilitated diffusion can occur in two different ways. The first is via channel proteins. These channel proteins resemble fluid filled tubes through which the solutes can move down their concentration gradients across the membrane. These channels are often responsible for assisting ions such as Na+, K+, Ca2+ and Cl-cross the membranes. Even though they are open tubes, they are often very specific for the ions that pass through them. For instance, a K+ channel may allow K+ to pass through, but not Na+ or Cl-. Also, as we shall learn later, the regulation of the movement of the various ions across the membranes is crucial for many important cellular functions. These channels, therefore, are often gated (they have doors or gates that can be opened or closed). Depending on the channel, these gates may respond to voltage difference across the membrane (voltage-gated channels), specific signal molecules (ligand-gated channels), or even stretching or compressing the membrane (mechanically-gated channels).
The second type of facilitated diffusion utilizes carrier proteins in the membrane. Unlike the channel proteins, these carriers do not open to both sides of the membrane simultaneously. Instead, they bind to a specific solute on one side of the membrane. This binding causes the carrier to change shape, which moves the solute to the other side of the membrane (think of a revolving door). Like the channel proteins, these carriers can be very specific for the solute they transport, since the solute must bind to a receptor site that is designed to fit the specific solute. Another interesting characteristic of these carriers is that they have a maximum rate of transport and can thus become saturated if the solute concentration is high enough. More on this later. An important family of carrier proteins transport glucose across cell membranes. To date, 12 carriers in this family have been identified. They are simply identified as GLUT1 through GLUT12. Their distribution and specificity varies. For example, GLUT2 is found in the liver and pancreatic islets while GLUT4 is found in skeletal muscle and fat tissue. Interestingly, GLUT4 is the only one of these carriers that requires insulin for maximal activity. As mentioned above, one of the characteristics of carrier proteins is that they can become saturated. One of the symptoms of uncontrolled diabetes is the presence of glucose in the urine. This is due to the fact that so much glucose is entering the kidney tubules that the transporters that normally move the glucose back into the blood become saturated, and the excess glucose ends up in the urine. Below are two links to videos that demonstrate the properties of the passive processes we have been discussing.
http://www.youtube.com/watch?v=GFCcnxgXOhY (Transcription Available)
http://www.youtube.com/watch?v=JShwXBWGMyY&NR=1 (Transcription Available)
Active Transport
To this point, the transport processes we have discussed have all been passive processes in which the solute, or the water movement has been down a concentration gradient with no input of energy required. However, there are times when it is important for the cell to be able to move solutes against their concentration gradient. Just like moving water from the first to the top floor of a high-rise building these processes require an energy source. Processes that require energy are called active transport processes.
Primary Active Transport
Primary active transport can move solutes, such as ions against their concentration gradient. This process requires a carrier protein that is much like the proteins involved in carrier-mediated diffusion mentioned above. However, in this case, the carrier has a site for the binding of ATP, which provides the energy to move the solute against its gradient. These transport systems can move one, or multiple ions across the membrane. One of the most important active transport systems is the Na+, K+-ATPase (see figure below). This system moves sodium out of the cell and moves potassium into the cell. Each cycle of the pump moves 3 sodium ions out and 2 potassium ions into the cell. Potassium is the primary intracellular cation in the body while sodium is the primary extracellular cation and Na+,K+-ATPase is responsible for maintaining this distribution.
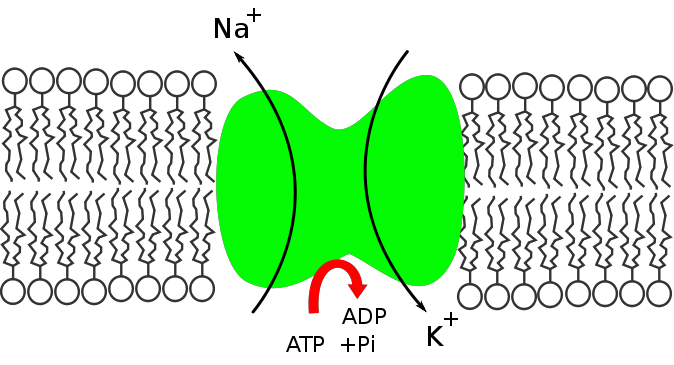
Image created at BYU-I by MG, 2013 .
Na+,K+-ATPase pumps 3 Na+ ions out of the cell and 2 K+ ions into the cell with the aid of ATP.
Secondary Active Transport
Like primary active transport, secondary active transport also moves solutes against their concentration gradients. However, with secondary active transport, ATP is not directly involved in the pumping of the solute. Instead, this process uses the energy stored in concentration gradients to move the solute. Since sodium is always in higher concentration outside of the cell (due to primary active transport), the sodium gradient is often used to power secondary active transport. In this process, the carrier protein has a binding site for the solute to be transported as well as a binding site for sodium. Once both solutes have bound, sodium moves down its concentration gradient and moves into the cell, much like what happens with carrier-mediated diffusion, and in the process pulls the other solute into the cell (symport), or moves it out of the cell (antiport), against its concentration gradient. A number of organic molecules are transported across membranes by this process, such as glucose and amino acids. ATP energy is required to generate the sodium concentration gradient, but is not directly involved in moving the desired solute across the membrane; hence the designation as secondary active transport.
Bulk Transport
To this point we have been talking about the movement of relatively small solutes across the cell membranes, i.e. ions and small organic molecules. There are instances, however, when it is necessary to move much larger materials across the membrane, such as when a macrophage engulfs a bacterium, or when larger amounts of a given material are released from a cell, such as the release of a hormone. These processes also require ATP and are therefore examples of active transport, but they move materials in a very different way.
Endocytosis is the bulk transport of material into the cell. There are several types of endocytosis and we will briefly explore each one. First let's discuss phagocytosis (see figure below), which means "cell eating." Only a limited number of cells are capable of phagocytosis, specifically cells of the immune system. In this process the cell sends extensions of its plasma membrane, called pseudopodia out and around the particle to be phagocytized. As these pseudopodia surround the particle, they eventually fuse, creating a vesicle containing the particle. This phagosome can then unite with a lysosome inside the cell and the engulfed material can be digested for use within the cell. Watch an amoeba phagocytize a paramecium in this clip http://www.youtube.com/watch?v=aWItglvTiLc. (Transcription Available)
In this next clip watch a neutrophil, one of our white blood cells, chase down and phagocytize a bacterium. http://www.youtube.com/watch?v=VAhM9OxZDkU&feature=related
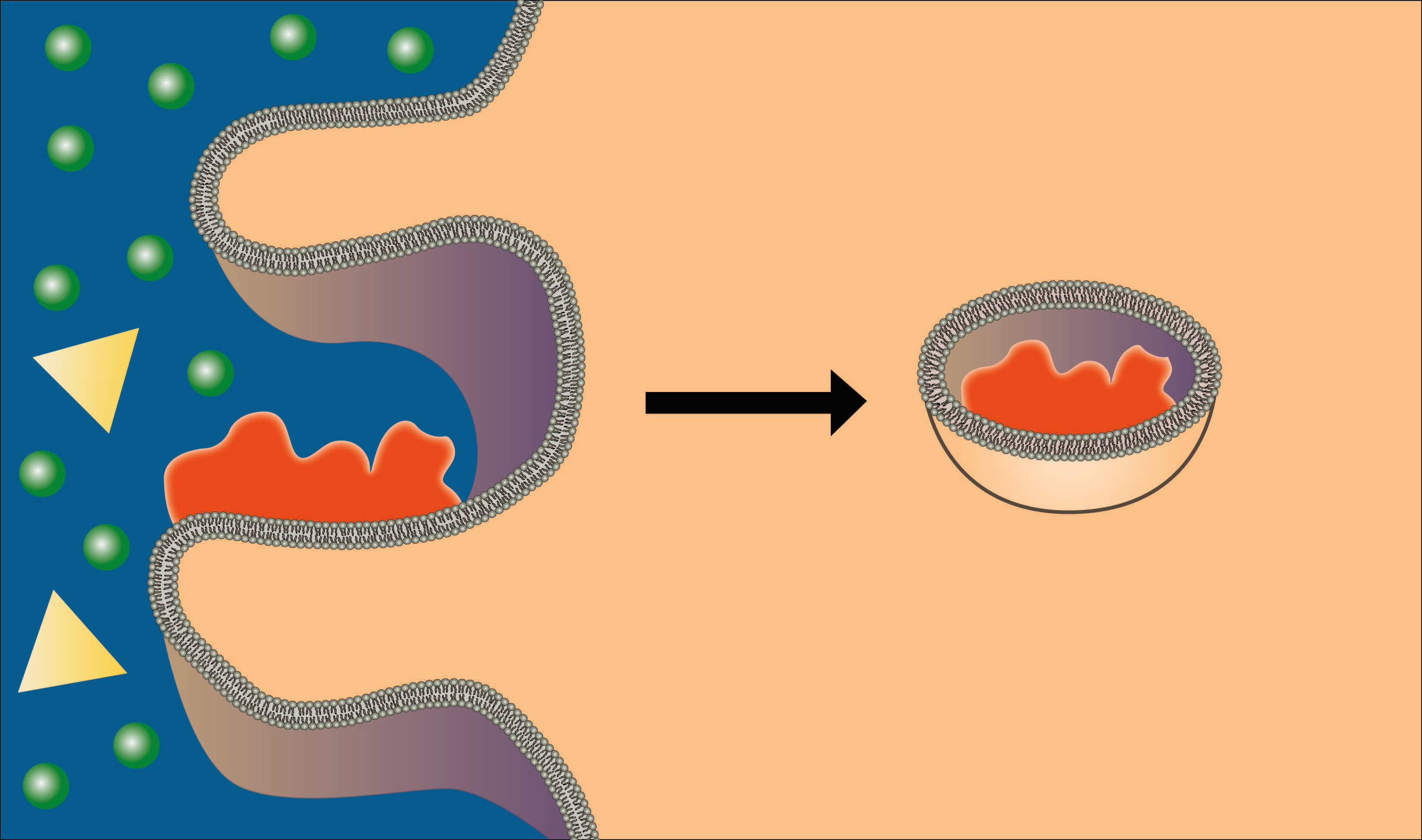
Image created by BYU-I student, Hannah Crowder, 2013 .
In phagocytosis the cell membrane forms processes that surround and engulf a particle to be brought into the cell.
A second type of endocytosis is pinocytosis, or "cell drinking." In this process, rather than send out pseudopodia, the cell membrane simply invaginates, forms a pocket, and engulfs anything in the fluid that is taken into the cell (see figure below). Unlike phagocytosis, pinocytosis occurs in most cells of the body. The cells are not interested in the water in the vesicles, but any solutes that might be brought in. As you can imagine, this is not a very efficient way of bringing materials into the cell because it is nonspecific and brings whatever is in the fluid into the cell. It provides cells with a nonselective mechanism for sampling the extracellular environment. It is prominent in cells involved in moving large amounts of material across the membrane, like cells of the intestines and the kidneys.
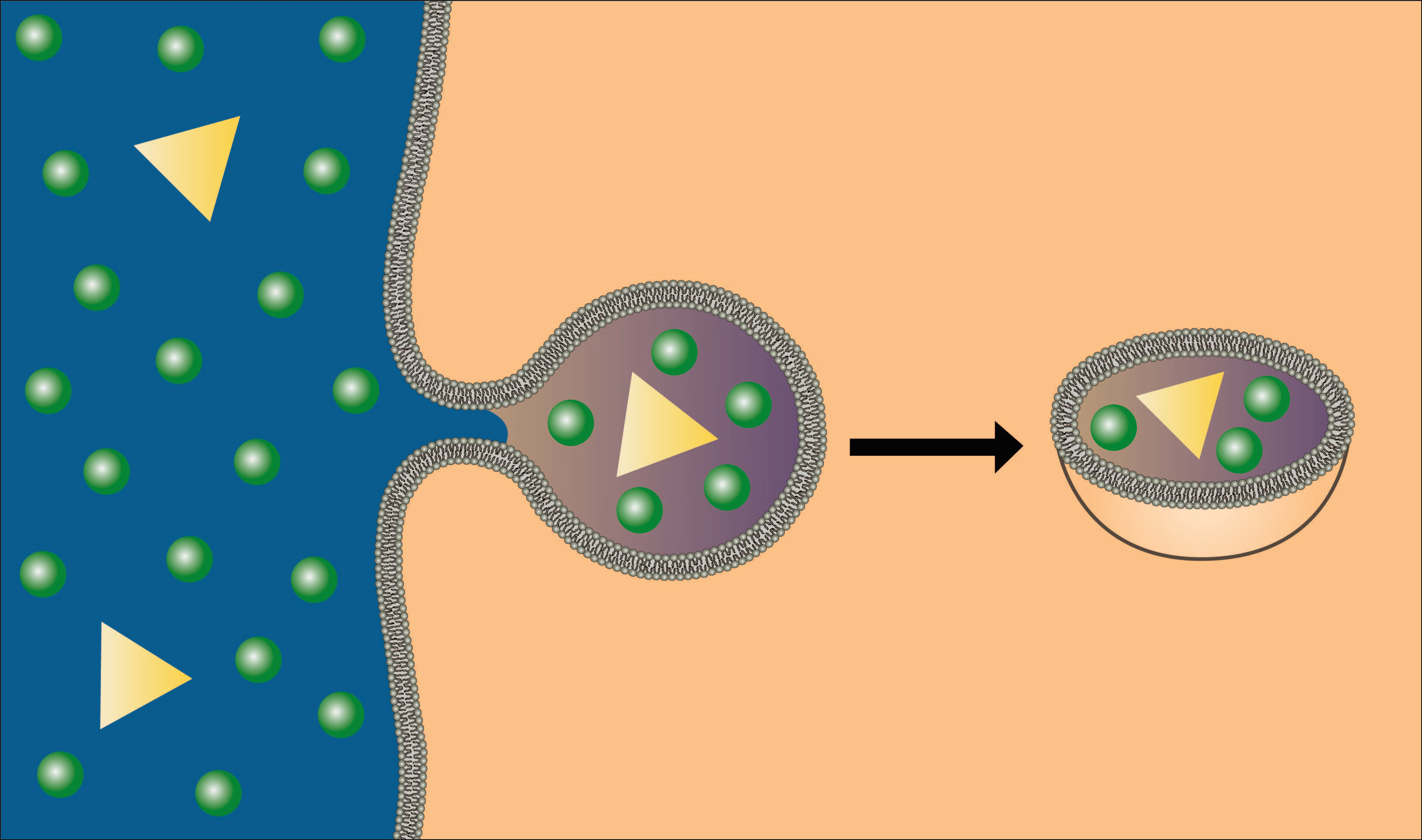
Image created by BYU-I student, Hannah Crowder, 2013 .
In pinocytosis the membrane forms an invagination (pocket) that pinches off, bringing into the cell the fluid in the pocket along with any solutes in the fluid.
A much more efficient mechanism for bringing specific solutes into the cell is receptor‐mediated endocytosis. As the name implies, this mechanism employs specific receptors that bind to the material (ligand) to be brought into the cell. Once the material binds, the receptor‐ligand complex migrates to a specific area of the membrane, a clathrin‐coated pit, which is then brought into the cell by a process similar to pinocytosis (see figure below). The advantage of receptor‐mediated endocytosis is that it can engulf large amounts of a specific solute. The following two animations demonstrate how this process occurs. The first is a general mechanism for receptor mediated endocytosis and the second shows how a specific molecule, cholesterol, is brought into the cell by this process.
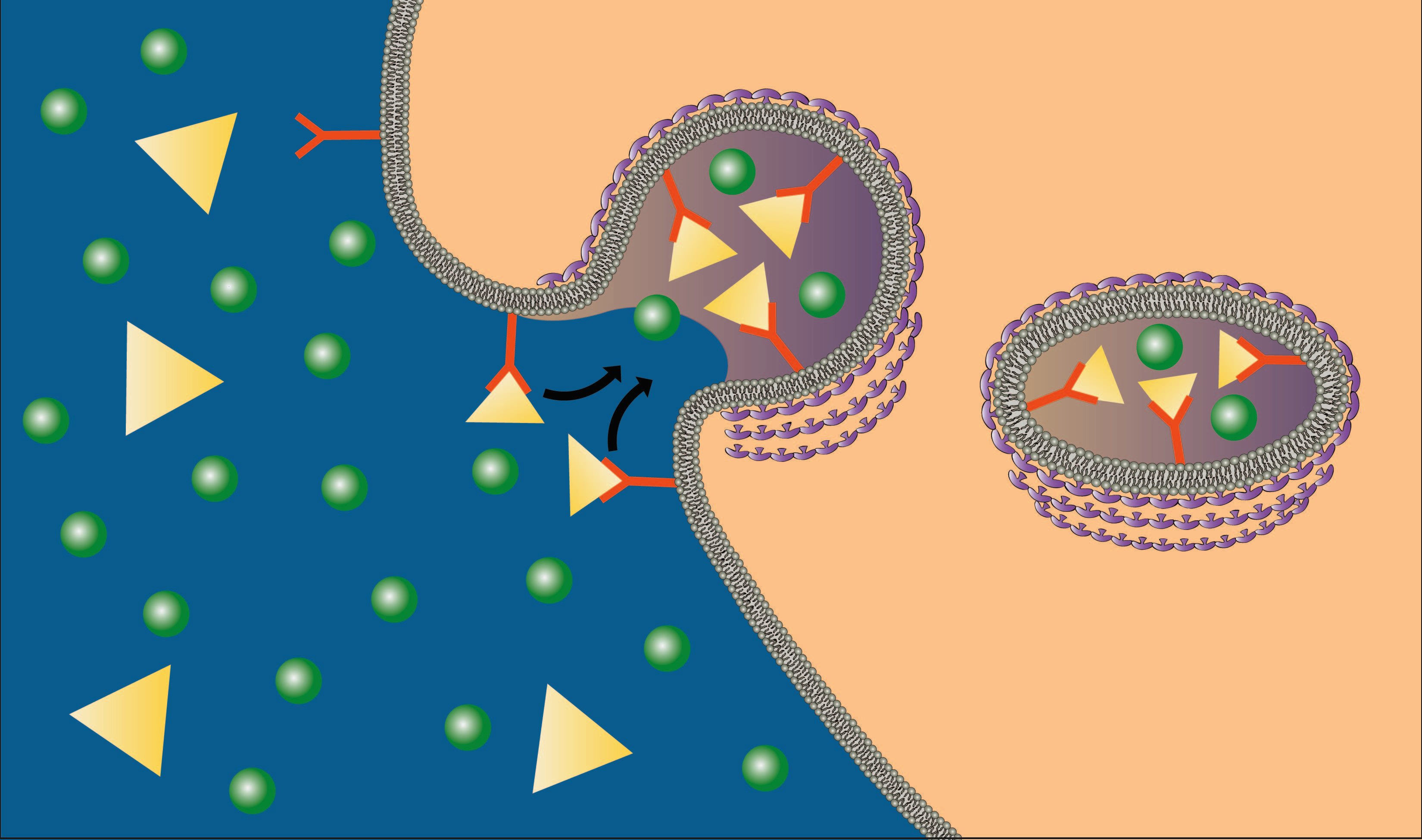
Image created by BYU-I student, Hannah Crowder, 2013 .
In receptor mediated endocytosis, ligands bind to specific receptors, which then migrate to a clathrin‐coated pit. The contents are then brought into the cell by a process similar to pinocytosis.
Thus far we have been discussing bulk transport, bringing material into the cell. There is also a need to export material from the cell into the extracellular fluid. This process is called exocytosis. For example, exocytosis is the process by which the beta cells of the pancreatic islets secrete insulin into the extracellular fluids. The mechanism is essentially the reverse of endocytosis. Secretory vesicles filled with the material to be released migrate to the plasma membrane where the membrane of the vesicle fuses with, and actually becomes part of the plasma membrane (see figure below). The material that was in the vesicle suddenly finds itself outside of the cell. The usual signal that initiates this process is the entry of calcium ions into the cell. Recall that calcium is an extracellular ion, so there is a large diffusion gradient for calcium to move into the cell. The opening of gated calcium ions, allowing calcium to diffuse into the cell, initiates the exocytosis process. The links below show how this might work.
http://www.youtube.com/watch?v=4gLtk8Yc1Zc (Transcription Available)
http://www.youtube.com/watch?v=K7yku3sa4Y8&NR=1
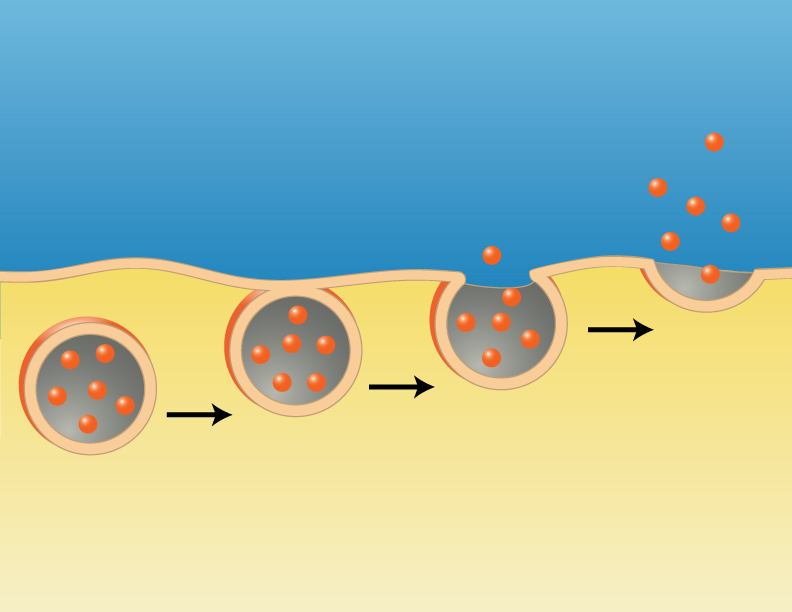
Image created by BYU-I student, Hannah Crowder, 2013 .
In exocytosis, secretory vesicles migrate to the cell membrane where the vesicular membrane fuses with the plasma membrane, releasing the vesicles contents into the extracellular fluid.
**You may use the buttons below to go to the next or previous reading in this Module**


