CELL MEMBRANES-STRUCTURE AND TRANSPORT
OSMOSIS
A special type of passive transport is the movement of water across a membrane, or osmosis. By definition, osmosis is the diffusion of water through a selectively permeable membrane from an area of high water potential (low solute concentration) to and area of low water potential (high solute concentration). Therefore, for osmosis to occur, the membrane must be permeable to water, but impermeable to the solute, and the concentration of the solute must be different on the two sides of the membrane. Water will move from the side with lower solute concentration to the side with higher solute concentration until the concentrations are equal, or until some external force prevents further movement of water. This is a passive process, in that no energy expenditure is required for the movement of water. In an artificial system such as the one depicted in the figure below, water will attempt to move from chamber B to chamber A. Since chamber A is a rigid chamber, pressure will develop. The pressure that is just sufficient to prevent water from moving across the membrane is referred to as osmotic pressure.
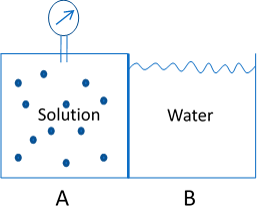
Image by BYU-I student, Hannah Crowder, 2013.
Movement of water from chamber B to chamber A will generate a pressure known as osmotic pressure.
In the body, water will move into or out of cells, depending on the solute concentration (osmolarity) of the extracellular fluids vs. the intracellular fluids. If the solute concentration in the extracellular fluid is lower than the solute concentration in the cell, water moves into the cell and the cell will swell. Before we can explain why cells shrink or expand when placed in a certain kind of solution, we first need to discuss the difference between osmolarity and tonicity.
Earlier this semester we discussed how the concentration of particles in a solution is expressed as osmolarity. Recall that osmlarity represents the number of moles of particles per liter of solution, while molarity represents the number of moles of molecules per liter of solution. Why do we have these different ways of expressing concentration? We have to change because different substances behave differently in solution. For example, when NaCl is dissolved in water, it breaks apart into Na+ and Cl- ions (this is a characteristic of substances held together by ionic bonds). Thus, there are now twice as many particles than there were when the substance was dry. Consequently a 1 molar solution of NaCl would be a 2 osmolar solution. Glucose is different. Glucose doesn't break apart in water because the atoms are covalently bonded. Therefore, a 1 molar solution of glucose will also be a 1 osmolar solution. The concentration of solutes in body fluids is 285-295 mOsmoles/liter (for simplicity we often round this number to 300). We place the small m, which stands for milli or one-housandth, in front of osmole because we are dealing with very small amounts, 1000 times less than an osmol. Osmolarity is a useful term because now we can use words to describe solutions like isosmotic, which means two solutions have the same number of particles, or hyperosmotic which means one solution is more concentrated than the other, or hyposmotic which means that one solution is less concentrated than the other. (Note: Osmolarity takes into account all of the particles in the solution. Therefore, if you have a liter of solution containing one mole of glucose and one mole of NaCl you would have a three osmolar solution.)
Perhaps the most important concept when talking about solutions and how they affect the body is tonicity. Tonicity (G. tonus, tone = firmness or stretch of a tissue) is a term used to describe how a solution affects a cell when it is placed into the solution. One important characteristic is that now we begin to deal with membranes and particles. Why are cells affected by different solutions? The answer lies in the behavior of particles with regard to diffusion. Particles will tend to diffuse from areas of high concentration to areas of lower concentration to reach equilibrium. However, if the membrane is not permeable to the particles, then instead of particles moving or diffusing, water will move or diffuse through the cell membrane to reach equilibrium. Additionally, at equilibrium, the osmolarities of the two solutions will be the same. We call the movement or diffusion of water osmosis, as was mentioned above. When water moves out of a cell, the cell shrinks; likewise, when water moves into a cell, the cell swells. Thus, if we place a cell into an isotonic solution, the cell shape won't change because the solutions are already in equilibrium, so there will be no net movement of water or solutes across the membrane. In other words, isotonic solutions have the same concentration of osmotically active particles (osmotically particles are non-permeable particles) as are found in the cell. If the cell swells, we say that the solution was hypotonic, and if the cell shrinks (crenates) we say the solution was hypertonic.
It seems simple enough. Water moves when the particles are impermeable to the membrane; however, when particles are permeable, the particles will diffuse through the cell membranes, instead of water. Remember the intracellular and extracellular fluids in the body always move to equilibrium, either by movement of solutes, if they can cross the membrane, or by the movement of water, if the solutes cannot cross. Let's look at another example. Five percent dextrose (dextrose is another name for glucose) is isosmotic with regard to body fluids because it has the same number of particles as blood; however, glucose is permeable to cell membranes. Thus, a 5% dextrose solution (D5W) may be isosmotic to the cells, but it behaves as a hypotonic solution—the solute moves into the cells accompanied by water, causing the cells to swell. Here is another way to think of osmolarity and tonicity; osmolarity can be used to compare the concentration of solutes in two solutions. It can also be used to compare the concentration of the solutes in a solution with those in the cell, before equilibrium is achieved. Tonicity is used to describe what effect the solution has on the cell. Osmolarity doesn't take into account the nature of the solutes while tonicity is dependent upon the concentration of the nonpermeable solutes.
The figure below shows what happens to red blood cells when they are placed into hypertonic, isotonic or hypotonic solutions.
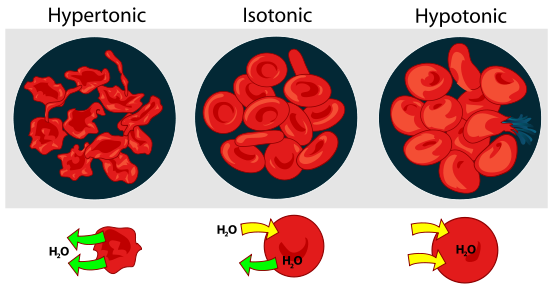
Title: File:Osmotic pressure on blood cells diagram.svg; Author: LadyofHats; Site: https://commons.wikimedia.org/wiki/File:Osmotic_pressure_on_blood_cells_diagram.svg; License: Public Domain
When placed in a hypertonic solution, red blood cells will shrink or crenate. When placed in an isotonic solution, there will be no change in volume, and when placed in a hypotonic solution, they will swell. If the concentration of the solution is great enough, they will burst (lyse).
The link below shows what happens to a wilted plant when it is placed into a hypotonic solution.
http://www.youtube.com/watch?v=H6N1IiJTmnc
To check our understanding, complete the table below by filling in the missing column items with regard to osmolarity and tonicity. Use the terms iso, hypo, and hyper to complete the table.
|
SOLUTION |
OSMOLARITY |
TONICITY |
|
0.9 % Saline |
||
|
5% dextrose |
||
|
5% dextrose + 0.9% Saline |
||
|
0.45% Saline |
||
|
5% dextrose + 0.45% Saline |
You have been given the answers for the table above. Be sure you understand why the answers are what they are.
**Note: Other texts, even hospitals on occasion, tend to use less rigorous definitions of tonicity. In other words, definitions are loosely given to define all hyperosmolar solutions as hypertonic. This is based on the observation that water can cross the membrane faster than the permeable solute can cross. It may also be based on the incorrect assumption that tonicity and osmolarity are the same thing. Thus, the initial effect of an abrupt change in extracellular osmolality may be temporarily different from the predicted tonicity change.
In other words, a 5% dextrose solution in saline would be considered hypertonic because there are more than 300 mosm of solutes in this solution. The problem with this definition is that it does not distinguish tonicity from osmolarity, as it makes no reference of whether the solutes are permeant or non-permeant, with respect to a particular membrane. We believe that loose definitions create confusion and therefore have defined tonicity by the more rigorous definition of "effective osmolarity."
**You may use the buttons below to go to the next or previous reading in this Module**


