ORGANIC CHEMISTRY
LIPIDS
In the Arctic region of the northern hemisphere is a group of people that make up the Inuit culture. The Inuit people have tragically adopted much of the American diet that we indulge in (fast food, sugary drinks, processed meals, etc.). As a result, the Inuit incidence of diabetes and cardiovascular disease is rising dramatically. Inuit natives who adhere to the traditional diet of marine mammals, fish, with some berries and greens, are not demonstrating the startling trend of metabolic disease.
Some call this the Inuit Paradox. The paradox refers to the fact that traditional Inuit diets consist of large amounts of fat. Whale fat, seal fat, caribou fat, and other small animal fat is regularly consumed as a staple. In fact, the daily fat consumption is nearly two times the recommended daily allowance published by the health and nutrition experts in our government. So, why can the Inuit people exist on double the recommended dose of fat intake and actually decrease their incidence of cardiovascular disease and diabetes? This would seem to be a paradox.
There must be more to the unhealthy American diet than the amount of fat consumed. It appears that the type of fat consumed is at least as important as the quantity. Have you heard people talk about saturated and unsaturated fat, or vegetable oil and animal fat? Have you heard anything about omega 3 oils and omega 6 oils? What about trans fats and cholesterol? It seems that not all fat and oils are the same. Perhaps the types, mixtures, and ratios of fat and oils found in the traditional Inuit diet can help explain the health benefits enjoyed by traditional Inuit natives. Much is being printed these days about the types of fat we eat and how to choose them wisely.
Science is taking a closer look at other traditional diets as well. Have you heard the hype about the heart protective effects of the Mediterranean diet? Some feel that the types of oils and fats found in this part of the world may have heart protective effects. You will likely hear and read more on the topics of fats and oils in years to come. In order for you to be a literate consumer of this information, it will be important that you know your fats. This section will teach about the different types of lipids. You will learn some of the basic chemical properties and categorizations for oils and fats.
THE NATURE OF LIPIDS
The lipids include a vast array of naturally occurring organic molecules. Lipid molecules give us fats, oils, waxes, cholesterol, cell membranes, some pigments, some vitamins, and many other important compounds. Among the many types of lipids, the terms "fat" and "oil" are probably the most familiar. Fats are generally solid at room temperature while Oils are a liquid.
Lipid of any type contains the same structural elements as carbohydrates (Carbon, Hydrogen, and Oxygen). However, in lipids, the ratio of hydrogen to oxygen is considerably greater. For example, the common lipid, stearin, has as many as 18 hydrogen atoms for every oxygen (see figure below).
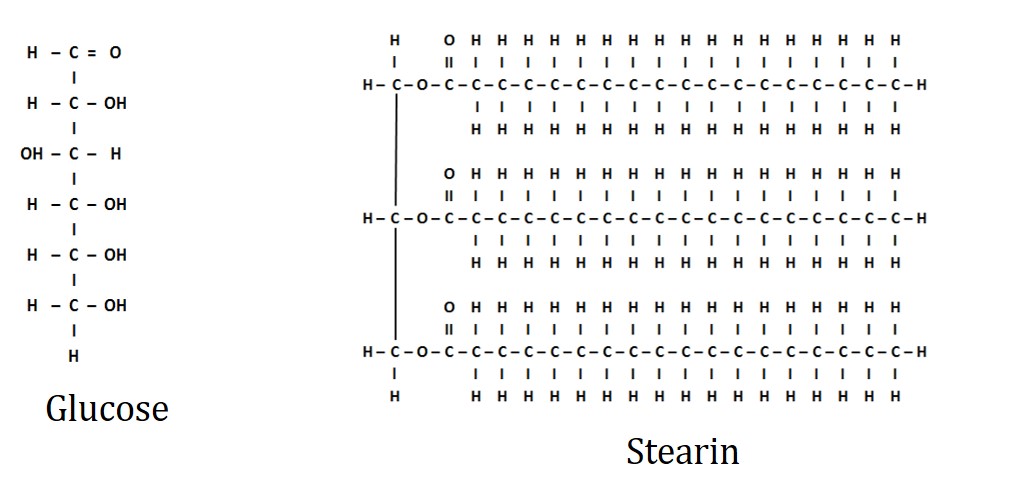
Image created by JS at BYU-I; 2014
Line drawings of a glucose and stearin molecule. Stearin is a triglyceride saturated fat that is commonly found in animal products.
The many carbon-hydrogen bonds in a lipid are referred to as a "hydrocarbon chain." Hydrocarbon chains are nonpolar, so do not interact well with polar substances like water. This is why we often say that "oil and water do not mix".
TRIGLYCERIDES
Triglycerides (also called triacylglycerols) constitute the major form of fat stored in plants and animals. Over 90% of our dietary fat exists as triglycerides and over 90% of the fat in a human body resides as triglycerides in adipose tissue below our skin. It is this stored fat that helps cushion and insulate the body. It is also this stored fat that can become excessive and contribute to the many problems of obesity.
Triglycerides are composed of two molecular building blocks-glycerol and fatty acid. As seen in the figure below, a glycerol molecule is connected to three fatty acids. Fatty acids are hydrocarbon chains with a "-COOH" group at one end. It is the fact that the "-COOH" group can lose the hydrogen as an H+ that gives the characteristic of an "acid" to these hydrocarbon chains. Each fatty acid bonds to the glycerol through a dehydration synthesis reaction. Note that the glycerol is always the same, while the fatty acid chains can vary in length as well as the number and type of double bonds.
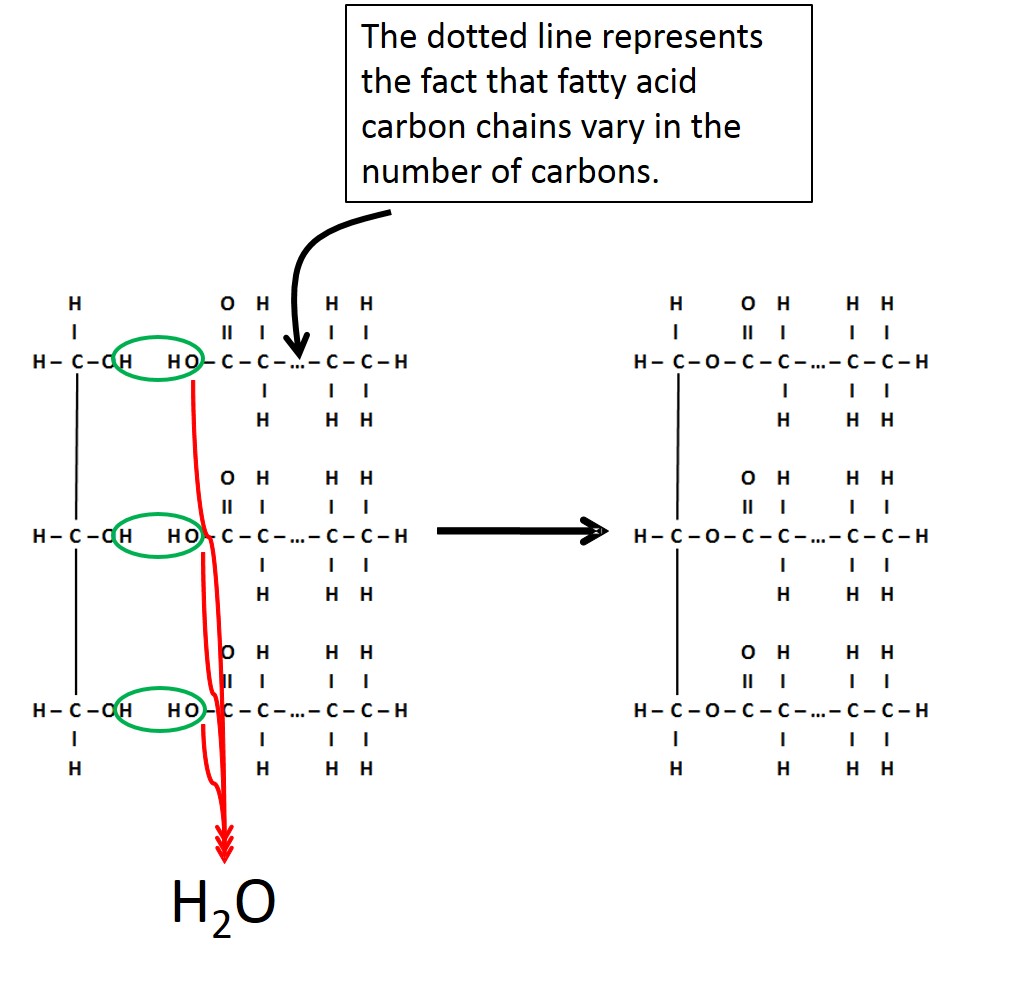
Image created by JS at BYU-I; 2014
A triglyceride is formed by the bonding of 3 fatty acid molecules to a glycerol molecule. This bonding occurs through a dehydration synthesis reaction.
Fatty acid chains with no double bonds are referred to as saturated. This means that every carbon-carbon bond in the chain is a single bond, which allows the linking of 2 hydrogen atoms to every carbon in the chain and 3 hydrogen atoms bonded to the last carbon.
If a double bond occurs between two carbons in the hydrocarbon chain, then the carbon atoms connected by a double bond will each bond with one less hydrogen atom in order to maintain 4 bonds per carbon atom. We could say that because of the double bond, the fatty acid hydrocarbon chain is no longer "saturated" with hydrogen atoms at every carbon. Therefore, an unsaturated fatty acid will contain one or more double bonds (see the image below). A fatty acid with one double bond is referred to as a monounsaturated fat, and fatty acids with two or more double bonds are polyunsaturated fats. All lipid containing foods have a specific mixture of saturated and unsaturated fatty acids. Saturated fatty acids tend to be straight and do not have "kinks" or angles that would make "packing" or "stacking" together more difficult. The more tightly packed molecules of fat become, they are more likely to be a solid at room temperature. Unsaturated fats can have either "cis" or "trans" double bonds in the hydrocarbon chains. "Cis" bonds allow for a kinked or angled geometry that makes it more difficult to "pack" together.
Unsaturated fats with "Trans" bonds contain a geometry that resembles the straight line of a saturated fat. This geometry allows for trans unsaturated fats to pack together tightly enough that they will be found as a solid at room temperature (see figure below). Products like Crisco and Margarine often have substantial quantities of "trans fats". Cis fats are natural and found readily in nature. Trans fats are rare in nature and have appeared in the American diet as a product of oil processing. Food manufacturers will take oils with cis bonds and use high pressures, high temperatures, and hydrogen gas to artificially "hydrogenate" unsaturated fats. This process creates saturated fats but also creates fats with rearranged double bonds (trans fats). This type of fat is usually listed as partially "hydrogenated oil" in the food ingredients list.
Epidemiologic, clinical, and physiological studies are quite consistent in showing saturated and trans fats to have adverse effects on our health if they are eaten in excess. Throughout the world, trans-fats have been acknowledged as unhealthy and many countries have outlawed their use. Substituting unsaturated fat for saturated and trans fat has sometimes been shown to lower LDL cholesterol which may have a "heart healthy" affect. There is also mounting evidence that some unsaturated fats may have the ability to decrease chronic inflammatory responses in the body. Omega 3 fatty acids have come into the spotlight lately as they may help to do this. Omega 3 refers to the existence of a double bond 3 carbons in from the end of a fatty acid chain. You can explore more about the types of health risks and benefits thought to be associated with different types of fats by going to this website:
http://www.helpguide.org/life/healthy_diet_fats.htm
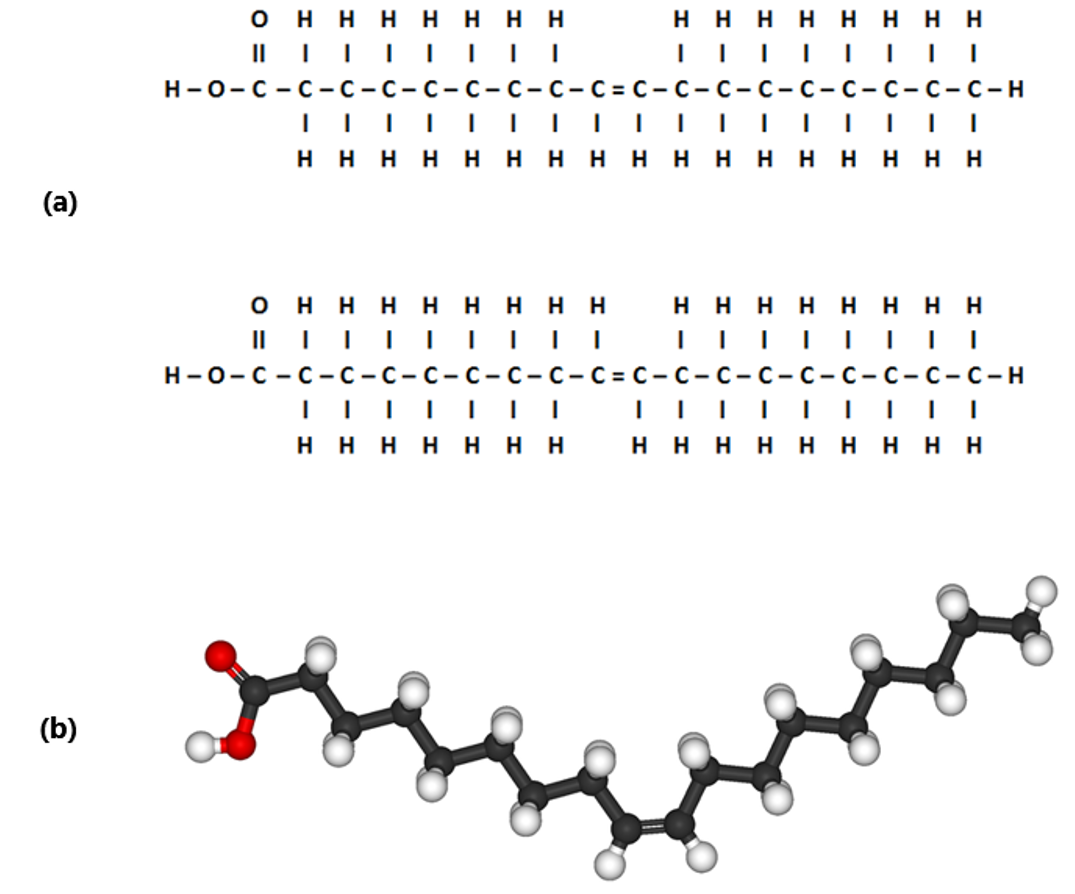
Image created by JS at BYU-I; 2014; ModifiedFile:Oleic-acid-3D-ball-&-stick.png; Author: Benjah-bmm27; Site: https://commons.wikimedia.org/wiki/File:Oleic-acid-3D-ball-%26-stick.png; License: Public Domain
This figure shows a line drawing of 2 monounsaturated fatty acids. (a) Note that the first molecule (a) has the hydrogen atoms extending from the carbon chain on the same side of the double bond. This is called a "cis" double bond (cis means same side). Note that the second molecule is nearly identical except that the hydrogen atoms extend from the carbons at the double bond on opposite sides. This is called a "trans" double bond (trans means to "cross"). (b) shows a 3D representation of the "cis" double bond in the unsaturated fatty acid chain. Notice how "cis" bonds bend or put a kink in carbon chain.
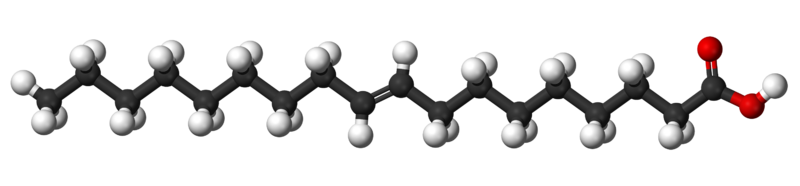
Title: File:Tridecylic-acid-3D-balls.png; Author: Jynto and Ben Mills; Site: https://commons.wikimedia.org/wiki/File:Tridecylic-acid-3D-balls.png; License: public domain
This figure shows an image of a double bond that is "trans." Notice how the "trans" double bond makes the fatty acid chain more linear. "Trans" double bonds make unsaturated fats more physically similar to saturated fats.
PHOSPHOLIPIDS
A phospholipid is structurally similar to a triglyceride. However, one of the three fatty acids are substituted for a polar phosphate group (see image below). Phospholipids are often referred to as "amphipathic," which means to have both a polar (hydrophilic or lipophobic) and non polar (hydrophobic or lipophilic) portion on the molecule. It is the amphipathic nature of phospholipids that allows them to maintain the structural integrity of a cell membrane and serve as a selectively permeable barrier that modulates movement of substances in and out of a cell.
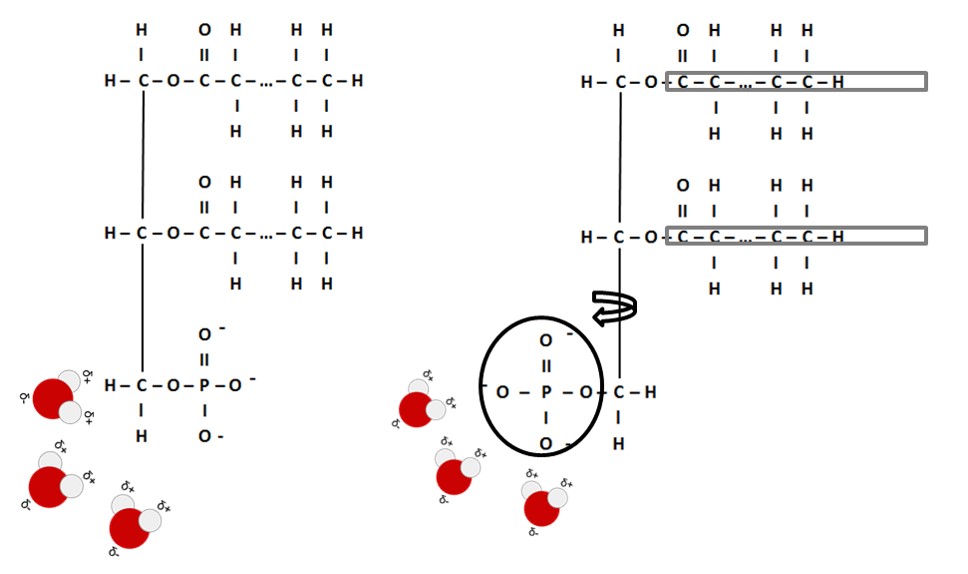
Image created by JS at BYU-I; 2014; modified File:Na+H2O.svg; Author: Taxman; Site: https://commons.wikimedia.org/wiki/File:Na%2BH2O.svg; License: Public Domain
A phospholipid is formed when an end fatty acid is replaced by a phosphate group. As the polar part of the phospholipid is attracted to water, the hydrophobic fatty acid tails are repulsed by water. The single carbon-carbon bond is flexible and allows the molecule to swivel such that the polar portion is with water and the hydrophobic tails are oriented away.
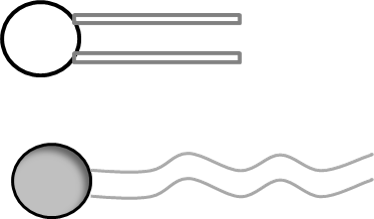
Image created by JS at BYU-I; 2014
It is common to represent a phospholipid by taking a circle (which represents the polar portion of a phospholipid) and attach it to two lines (which represent the hydrophobic portion of a phospholipid).
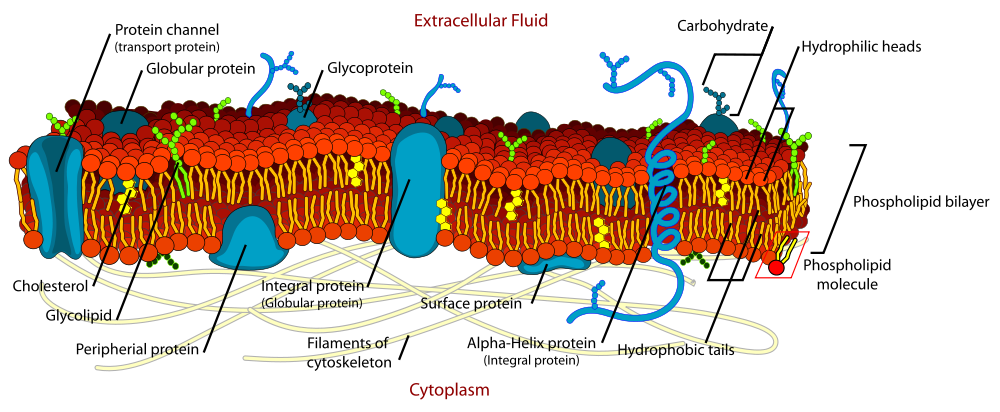
Title: File:Cell membrane detailed diagram en.svg; Author: LadyofHats Mariana Ruiz; Site: https://commons.wikimedia.org/wiki/File:Cell_membrane_detailed_diagram_en.svg; License: Public Domain
Phospholipids form a double layer or bilipid layer in cell membranes. The polar heads are oriented towards the aqueous cytoplasm and also towards the extracellular water. The fatty acid tails are oriented away from water but blend with each other. This configuration creates a barrier or boundary that separates the cytoplasm environment from the extracellular environment. This image also shows many of the other things found in cell membranes.
STEROIDS
A Steroid is a type of lipid that differs quite a lot in structure from the previous lipids discussed. Steroids have a characteristic arrangement of their hydrocarbon rings that are joined to each other (See image below). However, the solubility characteristics of steroids are like other lipids in that they are non-polar (hydrophobic). The best known and most abundant steroid in the body is Cholesterol. Cholesterol is very important as it is required to build and maintain cellular membranes. Cholesterol is used to synthesize bile, an important component of digestive juices that help in the digestion of fat. Cholesterol is also used to synthesize steroid hormones (see image below). Steroid hormones are critical for healthy growth and development of most tissues in our body. Cholesterol is essential to all animal life, so we find that animals (including humans) have the ability to make this important molecule. We can also ingest cholesterol. When we consume animal products, we obtain cholesterol to varying degrees. Cheese, egg yolks, beef, and pork are all examples of foods commonly considered to have substantial amounts of cholesterol. Cholesterol along with trans and saturated fats contribute to the formation of deposits on the inner walls of blood vessels. These deposits can become quite hard and can result in a condition known as atherosclerosis. In a later unit, we will present a chapter on atherosclerosis and talk in much more detail about this serious cardiovascular disease.
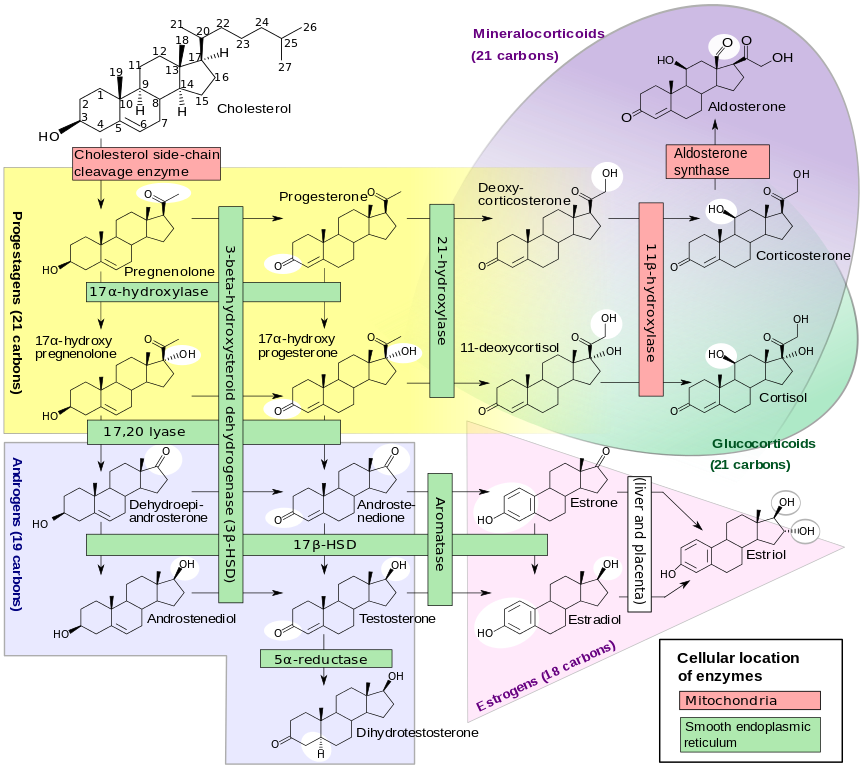
Title: File:Steroidogenesis.svg; Author: Hoffmeier and Settersr. Site: https://en.wikipedia.org/wiki/File:Steroidogenesis.svg; License: licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license.
This figure is rather complicated in its description of the many pathways that synthesize steroids from cholesterol. However, it is a good figure to help us appreciate the large number of steroid hormones that can come from cholesterol. You will likely find many names that you recognize (i.e. testosterone, estrogen, progesterone, cortisol).
LIPOPROTEINS
As you now know, lipids like triglycerides and cholesterol are hydrophobic. If you were to put these in water, they would not dissolve. This presents an interesting dilemma. Our blood vessels are the conduits that transport organic molecules to where they need to be in our bodies. Our blood is also mostly water. It seems that if we placed lipids in our blood, we would see a separation and droplets of fat and oil would separate from the water. This would not be good! A separation of lipid and water would make it difficult to distribute lipids evenly to all the necessary places, not to mention a lethal effect of having lipid droplets congregate and get stuck in very small vessels and blocking blood flow.
The solution to the lipid transport problem is the "Lipoprotein". The lipoprotein is an assembly of both lipids and proteins. This assembly will form a small particle that will have the hydrophilic ends of phospholipids and proteins pointing outward toward the aqueous plasma, while the hydrophobic ends will point towards the inside of this spherical particle. Cholesterol and Triglycerides are inside of these spheres and will be shielded from the water (See Figure below). This lipoprotein particle will dissolve in the water portion of blood plasma and be carried easily through the circulation. Lipids wont form droplets or plug up any vessels, because they are safely tucked away in the lipoprotein assembly. Cells throughout the body have the ability to bind these lipoproteins and move lipids in and out of them. There are several different types of lipoproteins and each one has a specific role. Some will focus on picking up lipids from the digestive tract, while others will specialize in the transport of lipids between the liver and body tissues. You may have heard the terms HDL and LDL. These are two common lipoproteins that have gained a lot of attention as they appear to correlate with the risk of atherosclerosis development.
Many brochures and websites refer to HDL as "Good Cholesterol," and LDL as "Bad Cholesterol." See this website for an example:
In reality, there is no such thing as "good cholesterol" or "bad cholesterol." Cholesterol is simply a type of lipid that we need and does not come in as "bad" or "good." The idea of "good" and "bad" refer to the lipoproteins. The more protein that a lipoprotein assembly contains, the heavier it is. LDL or Low Density Lipoprotein is an assembly of proteins and lipids that is lower in protein and higher in lipid content (See image below). LDL particles tend to accumulate in the walls of arteries. It is the overabundance of this LDL deposition that contributes to atherosclerosis, and thus the term "bad." HDL or High Density Lipoprotein is often called the "good cholesterol" because HDL particles help prevent atherosclerosis by extracting cholesterol from artery walls and disposing of it through biochemical reactions in the liver. HDLs also have relatively more proteins in the lipoprotein assembly, which gives it the name High Density (see image below). Research has shown that lowering LDL cholesterol reduces the risk of heart attacks, strokes, and atherosclerosis.
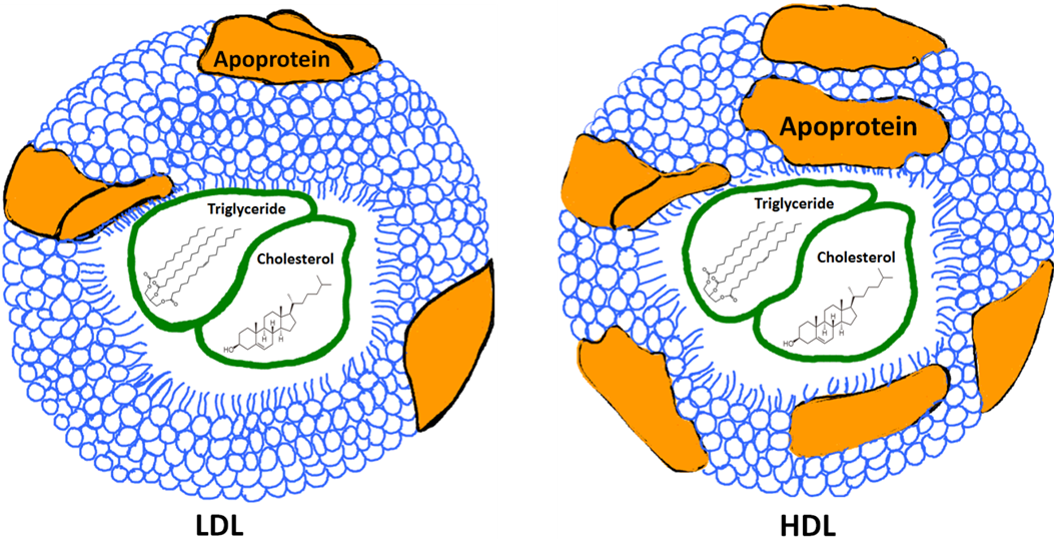
Image created by JS BYU-I; 2013.
A schematic representation of two types of lipoproteins. LDL stands for Low Density Lipoprotein. Lipoproteins are an assembly of phospholipids and proteins that carry triglycerides and cholesterol in the blood. Notice that the HDL or High Density Lipoprotein has more apoproteins (brown blobs) which increases this particle's overall density.
SUMMARY and LIPID PROFILE
The lipids include a large and diverse group of naturally occurring compounds. Triglycerides, phospholipids, and cholesterol are three important lipids in biology. The large stores of fat in our body are mostly triglycerides which also comprise the bulk of fat and oil that we consume. Triglycerides contain saturated and unsaturated fatty acids that lend physical and chemical properties such as solidity, texture, and flavor. Fatty acids also affect health. Saturated fatty acids appear to contribute to heart and vascular disease when consumed in high quantities. Trans fats also contribute to heart disease and are considered unhealthy to eat. Unsaturated fatty acids may actually lower some health risks like inflammation and heart disease.

Image Permission Aquired by email through Nate Wise BYU-I Dec 2013
LIPID PROFILE APPENDIX
The following appendix offers information regarding desirable and undesirable lipid profile values.
|
Total Cholesterol (TC) |
Category |
|
Less than 200 |
Desirable |
|
200-239 |
Mildly High |
|
240 and above |
High |
|
HDL |
HDL- Category |
|
60 and above |
High; Optimal; associated with lower risk |
|
Less than 40 in men and less than 50 in women |
Low; considered a risk factor for heart disease |
|
Triglycerides |
Triglyceride Category |
|
Less than 150 |
Normal |
|
150-199 |
Mildly High |
|
200-499 |
High |
|
500 or higher |
Very High |
|
LDL |
LDL Category |
|
Less than 100 |
Optimal |
|
100-129 |
Slightly Above Optimal |
|
130-159 |
Borderline High |
|
160-189 |
High |
|
190 and above |
Very High |
|
Non-HDL |
Non-HDL Category |
|
Non-HDL is a reading that includes the cholesterol content of all the lipoproteins that are not part of the HDL classification. LDLs are the most common lipoprotein to examine for heart disease risk, but there are other lipoproteins that can contribute to atherosclerosis. These are sometimes called Very Low Density Lipoproteins or (VLDL) and Intermediate Density Lipoprotein (IDL). The general category of all Non-HDL lipoproteins can be combined with the readings from the LDL category to validate concerns for heart risk profiles. |
|
|
Less than 130 |
Optimal |
|
129-159 |
Slightly above Optimal |
|
160-190 |
High |
|
Above 190 |
Very High |
|
TC/ HDL |
Category |
|
Total Cholesterol to HDL ratio (TC /HDL) is a number that reflects how many HDL lipoproteins we have relative to our total cholesterol. A person with a lower HDL value may see that the total cholesterol is also low. In this case, a person with a lower HDL value may have a TC/HDL ratio that is fine. This value taken with the other values in the lipid profile help a health care professional get a better idea of the actual heart disease risk. |
|
|
Below 3.5 |
Optimal |
|
3.6 to 5.0 |
Borderline High to High |
|
Above 5.0 |
High to Very High |
|
12 hour Fasting Glucose (Glu) |
Category |
|
82 - 110 |
Optimal |
|
111- 125 |
Borderline High to High |
|
Above 126 |
High to Diabetic |
**You may use the buttons below to go to the next or previous reading in this Module**


