ORGANIC CHEMISTRY
PROTEINS
Have you ever noticed that in virtually every organization there is always that one person who can do everything? No matter what needs to be done, that person somehow is able to do it. Think about that uncle in your family who is a "Jack-of-all-trades," who is always called on whenever there is a challenge or a need. Every baseball team has a utility player who can fill in at any position when he is needed. These individuals are indispensable to their organizations. The cell is no different. There is a need for a "utility" player in the cell. In the molecular world of the cell, who is the biological molecule that can do nearly everything? If you are guessing that it is the proteins, you are correct (of course the heading for this section may have tipped you off). Of the four classes of biological molecules, the proteins are the most diverse in their functions. By some estimates, our cells make more than 50,000 different proteins. Table 1 lists some if the major functions of proteins, but this list is not exhaustive, in fact it is hard to think of any function in the body in which proteins are not an integral part. In this unit we will learn about the molecular structure of proteins and discuss some of their important functions.
|
Function |
Example |
|---|---|
|
Structure |
Collagen in tendons and ligaments, Keratin in the nails and skin |
|
Transport |
Hemoglobin in the blood, Na+,K+-ATPase in cell membranes |
|
Protection |
Antibodies of the immune system |
|
Movement |
Actin and Myosin in muscles |
|
Enzymes |
Digestive enzymes in the small intestine (Lactase, Sucrase, Trypsin) |
|
Receptors |
Membrane proteins that respond to chemical messengers (insulin receptors) |
|
Regulation |
Chemical messengers: hormones, neurotransmitters, cytokines |
Amino Acids
Like polysaccharides and nucleic acids, proteins are polymers of smaller subunits or monomers. The monomers that make up proteins are the amino acids. Although there are 20 different amino acids that make up the proteins in humans, all have the same basic structure. Each has a central carbon with 4 diffrerent groups attached to it. The figure below shows the basic structure of an amino acid. Attached to the central carbon is an amine group (green) and a carboxyl group (red). Recall that a carboxyl group is an acid group. The name amino acid is derived from these two groups. Additionally, there is a hydrogen (blue), and finally, an R group. In organic chemists' shorthand, the R group represents some other organic group. In the case of the amino acids, there are 20 different R groups, hence, 20 diffrerent amino acids. It is the different R groups that confer the different properties to the amino acids. Some R groups are non-polar (hydrophobic), others are polar (hydrophilic). Some R groups are ions, either anions or cations (hydrophilic).
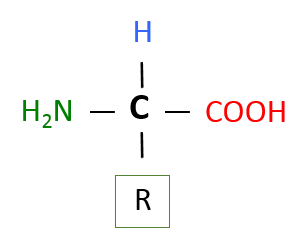
Image created by MG BYU-I; 2013.
The image above represents the basic structure of an amino acid. The central carbon (black) has 4 groups attached to it, an amine group (green), a carboxyl group (red), a hydrogen (blue), and one of 20 different R groups. Of an amino acid, "R" can be a variety of things, and in this image, it simply represents some type of organic group.
In this class you will not need to learn the names and structures of the individual amino acids, however, if you are interested in learning more about their structures and characteristics check out the following links:
http://www.aminoacidsguide.com/
http://en.wikipedia.org/wiki/Amino_acid
Peptide Bonds and Polypeptides
As mentioned in the introduction, proteins are polymers of amino acids. Like all of the polymers we have discussed so far, amino acids are linked together via dehydration (condensation) synthesis reactions. The bond that is formed between the amino acids is called a peptide bond. The figure below shows how these bonds are formed. In this simple example, we would call the resultant polymer a dipeptide. Small peptides are designited tripeptides, tetrapeptides, pentapeptides, etc. The generic term polypeptide is used to designate many amino acids linked together. The terms polypeptide and protein are often used interchangeably and there is no set rule for when each should be used. Some authorities suggest an amino acid cut off of 50 amino acids, anything less than 50 is called a polypeptide and anything over 50, a protein. Others use a molecular weight cut off with 10,000 being the division between polypeptides and proteins. Whether we call them proteins or polypeptides, it is of no real importance; they will still do their job no matter what we call them. Notice that the end amino acids in the polypeptide will have either an unbonded amine group or an unbonded carboxyl group. These ends are designated the amino- or N-Terminus and the carboxyl- or C-Terminus, respectively.
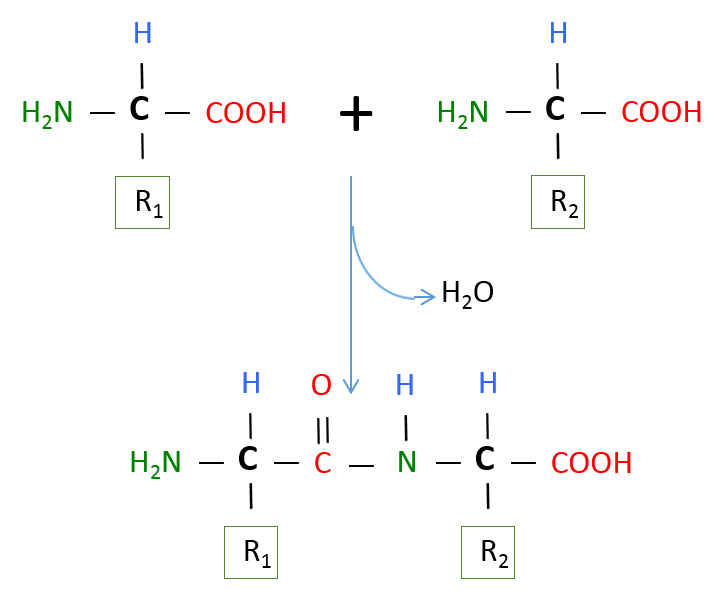
Image created by MG BYU-I; 2013.
The image above represents a dehydration synthesis reaction between 2 amino acids to form a peptide bond. Peptide bonds form between the carboxyl group of one amino acid and the amine group of another.
As mentioned above, almost all living things (except for a few rebellious strains of bacteria) contain proteins made from 20 amino acids. Our liver is a pretty effective amino acid factory, and can synthesize 11 of these 20 amino acids even if we don't consume them in our diet. However, nine amino acids are essential amino acids. If we don't consume these essential amino acids in our food, our body won't have the necessary supplies available when new proteins need to be produced.
For those of you who haven't already dozed off, let's reinforce these basic concepts. Think of amino acids as Lego blocks. If you were given 20 packages of these blocks, each package containing a different color, you could start producing Lego proteins. Just think of the possible combinations of your Legos. The possibilities are essentially limitless. Some of your proteins may contain only a few Legos while others may contain thousands. This is the potential that our cells have at their fingertips to produce the molecules to carry out the many functions of proteins.
Protein Structure
By now you should be starting to realize the importance of proteins to the proper functioning of the various systems in our bodies. What is it about proteins that allows them to perform all of these different tasks? The answer to this question can be summed up in three words: shape, shape, and shape. Let me say that one more time, a protein's function is determined by its shape. As you can imagine from the many functions of proteins, they have very complex shapes. If we think of proteins as cars, we all quickly understand that having the wheels on the bottom of the car and a steering wheel to guide the car are pretty important standard equipment. Similarly, if our protein "car" doesn't have the right parts in the right places with each component properly connected together, the protein does our body about as much good as a car that has been put through an auto crusher. In studying the shape of proteins, biochemists have dissected and broken them down into 4 levels of complexity or structure. As we move from the 1st to the 4th level of structure, the preceding level adds to the next. For example, you cannot have secondary structure without primary structure.
Primary structure: The primary structure of the protein is the sequence of the amino acids in its polypeptide chain. If proteins were popcorn stringers made to decorate a Christmas tree, the primary structure of a protein is the sequence in which various shapes and varieties of popped corn are strung together. The primary structure of a protein is maintained by covalent, peptide bonds connecting the amino acids together. The figure below shows the primary structure of insulin, the first protein to be sequenced. Notice on the right side of the figure the position of each amino acid is numbered. By convention, biochemists always number the amino acids beginning at the amino-terminus of the polypeptide chain.
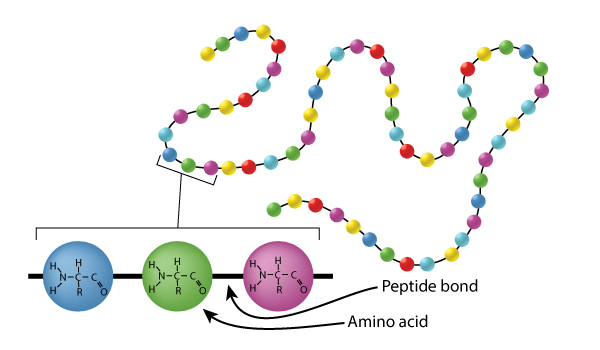
Image drawn by BYU-I student Nate Shoemaker Spring 2016
The image above represents the primary structure of a protein (a chain of amino acids). As you might expect, the sequence of the amino acids in the polypeptide chain is crucial for the proper functioning of the protein. This sequence is coded in the genetic code of the DNA. If there is mutation in the DNA and the amino acid sequence is altered, the function of the protein can be affected. All of the known genetic diseases, such as cystic fibrosis, sickle cell anemia, albinism, etc, are due to mutations that result in alterations in the primary structures of proteins, which then in turn, cause alterations in the secondary, tertiary and possibly quarternary structure.
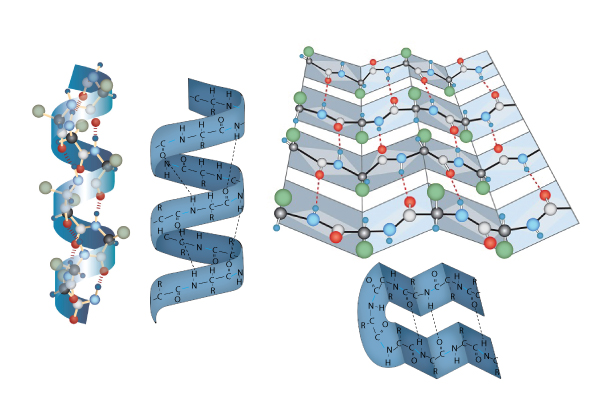
Image drawn by BYU-I student Nate Shoemaker Spring 2016
Secondary Structure: The secondary structure of proteins involves twisting or folding polypeptides into highly regular sub-structures. Whereas the primary structure of a protein is pretty much 2-dimensional, the secondary structure of proteins begins the very important 3-dimensional configuration of proteins.
The two types of secondary are the alpha helix (think "slinky" and to the left in the picture just above) and the beta pleated sheet, or simply pleated sheet (think about one of those folded cardboard windshield guards that can be placed on the inside of your car's windshield on a hot day so the inside of your car doesn't end up with a temperature approximately that of the interior of our sun. Found on the right in the picture just above). The secondary structure of proteins is a result of the sequence of amino acids in the primary structure and is maintained by hydrogen bonds. Some proteins, like collagen, are almost entirely alpha helix, while others, like silk, are mostly pleated sheet. Other proteins can have short segments of alpha helix and/or pleated sheet in their structure.
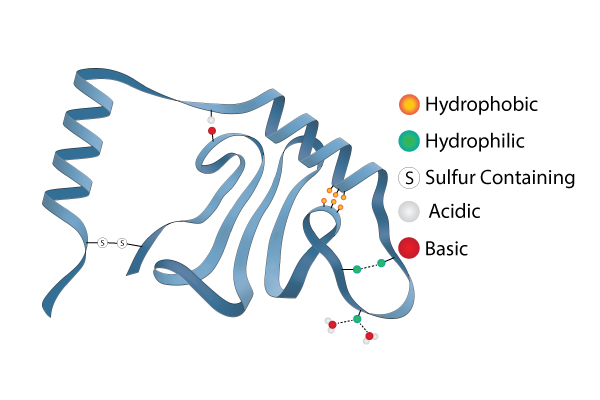
Image drawn by BYU-I student Nate Shoemaker Spring 2016
Tertiary Structure: The tertiary structure of a protein is the overall folding of the polypeptide chain. This folding involves more than just an alpha helix and a pleated sheet. There are other chemical interactions that can help define the 3 dimensional tertiary structure. Remember that the R groups of some amino acids are hydrophilic and others are hydrophobic. Since proteins in the body are in an aqueous solution, the hydrophilic R groups will tend to orient toward the water and the hydrophobic R groups will turn away from the water (towards the center of the protein). This can create some looping and folding in the protein structure. Sometimes hydrophilic groups will form ionic bonds or hydrogen bonds between different R groups. This helps stabilize the tertiary structure. Weak acids and bases can donate or accept protons in the aqueous solutions that proteins exist in. This can create positive and negative charges on the amio acids that will create attraction. pH can certainly affect how these attractions between acidic and basic R groups occur. This helps explain why radical changes in pH can cause the structures of proteins to fall apart and ruin the ability of the protein to function. This is called denaturing the protein. As a side note, very high temperatures can also cause some of these tertiary structure bonds to fail and is another way to denature a protein. One very important and very strong tertiary structure bond is actually a covalent bond that occurs between certain R groups (disulfide bonds between adjacent cysteine residues).
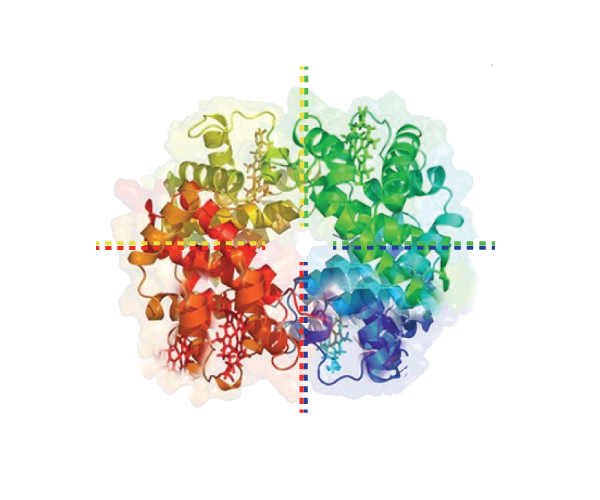
Image developed by BYU-I student Nate Shoemaker Spring 2016
Quaternary Structure: Not all proteins assume a quaternary structure. Only proteins composed of more than one polypeptide chain have quaternary structure. The protein in the picture just above, for example, has 4 polypeptide chains that work together to form one functional protein. A protein like the one in this picture that has 4 polypeptide chains (called subunits) that you might be familiar with is hemoglobin. Hemoglobin is found in your red blood cells and has a job of carrying oxygen throughout your body. There are 2 alpha and 2 beta chains that make up hemoglobin. You may have heard of sickle cell anemia? This is a mutation that results in a change to just one amino acid in the primary structure of the beta chains. But, this small change is enough to cause alterations to the secondary, tertiary and quaternary structure of hemoglobin. The alterations affect hemoglobin's ability to function correctly and a large array of pathological symptoms are the result.
This next image below is just a summary that shows all the levels of protein structure in one image. You can see how each level of structure leads to a more complex development of a very specific 3 dimensional protein.
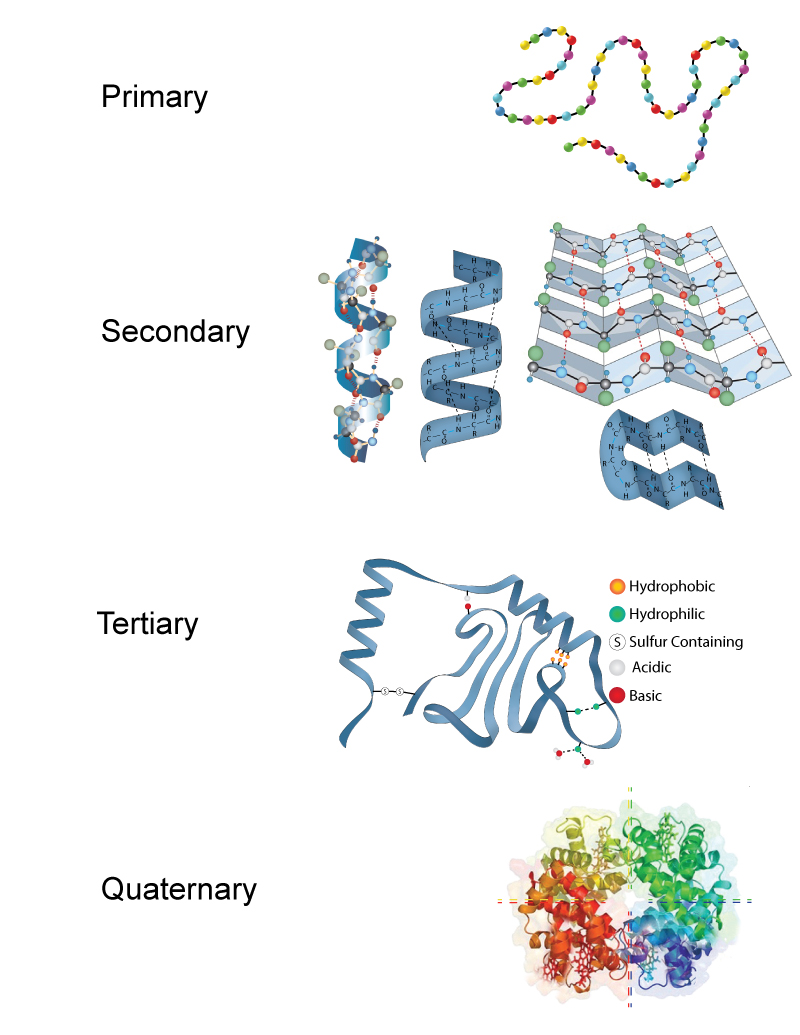
Image developed by BYU-I student Nate Shoemaker Spring 2016
Classes of proteins
There are two major classes or types of proteins: globular and fibrous proteins. Globular means globe-like. Hemoglobin is a good example of a globular protein. Globular proteins are quite fragile and can be inactivated (denatured) by things like heat (think of the protein albumin in an egg white when you fry it), organic solvents or strong ionic solutions.
Fibrous proteins are much stronger and tougher. As the name implies, these proteins are more like ropes or cables. We should all be grateful for fibrous proteins because if we didn't have them, we would all look like a melted milkshake on a hot sidewalk. Fibrous proteins give the body structural support and help it resist mechanical stress. Common examples of body structures containing fibrous proteins include bone, cartilage, tendons (which anchor muscles to bone), ligaments (which anchor bones to other bones) and capsules around our internal organs.
The two images below show the molecular image representations of first a globular and then a fibrous protein.
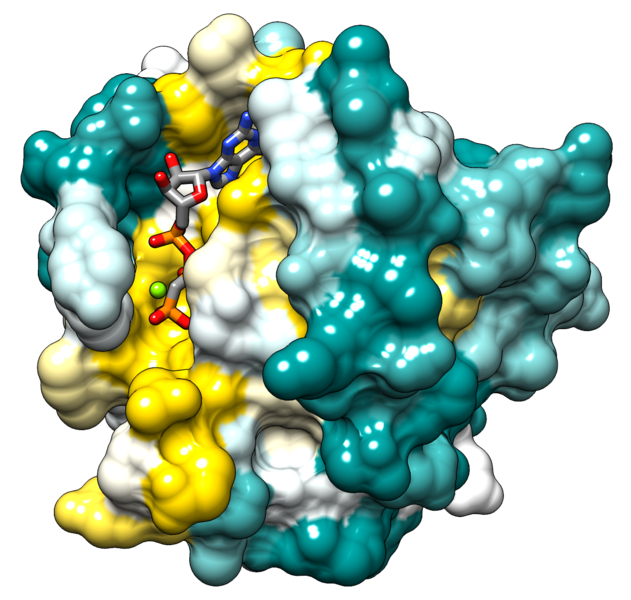
File:Hras surface colored by conservation.png; Author: ElaineMeng; Site: https://commons.wikimedia.org/wiki/File:Hras_surface_colored_by_conservation.png; License: licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license.
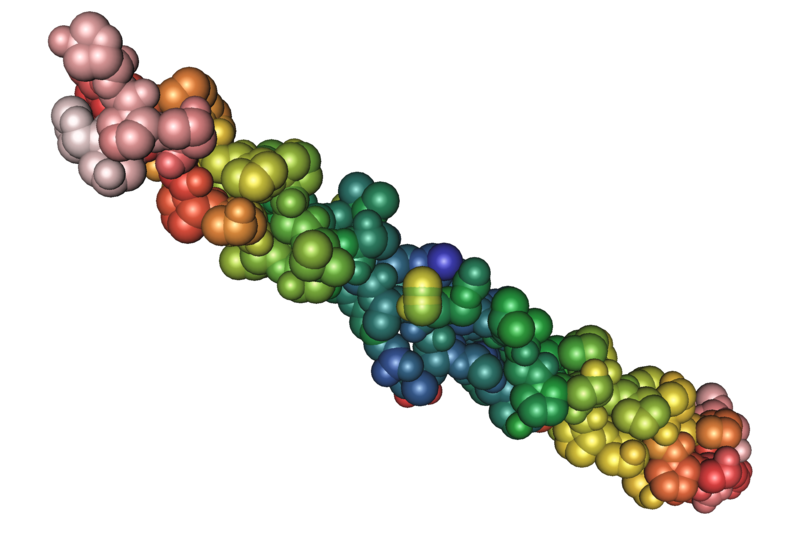
File:1bkv collagen 02.png; Author: Nevit Dilmen; Site: https://commons.wikimedia.org/wiki/File:1bkv_collagen_02.png; License: licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license.
Enzymes
One of the most diverse and important roles of proteins is that of enzymes. An enzyme’s purpose is basically to speed up the rate of a chemical reaction and they accomplish this by decreasing the activation energy needed to start the process. Let’s use a dating analogy to try and understand this ever important process!
Suppose a guy sees an attractive young lady and would like to take her on a date to get to know her better and perhaps even marry her. In order to start the dating/courting process there is a certain amount of courage or “activation energy” that he must invest. To just walk up and start talking to her might require more courage than he can muster and without some help he may never be able to initiate the dating process or “reaction.” In the chemical world this is where an enzyme satisfies a very important role and in the dating world this is where a mutual friend or matchmaker comes in very handy. Your mutual friend recognizes that you might be compatible and sets you up on a date…….remember that is what the guy wanted to do but was unable to muster the courage or “activation energy” to accomplish on his own. Now the hard part is over and the two individuals go on a date, yada yada yada, and emerge as one couple! There are a few very important things to keep in mind about enzymes:
- Enzymes are not used up or consumed during the chemical reaction. In other words a single enzyme can serve as a catalyst for one reaction after another. In the example above the matchmaker could go on to set up other dates between other young men and women.
- Enzymes are quite specific and so a single enzyme is able to catalyze a reaction between certain reactants (substrates) but not others, which is why we need so many different enzymes. An example that you are familiar with is converting a common disaccharide, sucrose, to two monosaccharides, glucose and fructose. The enzyme that is involved in this reaction would be unable to convert the disaccharide lactose to the monosaccharides glucose and galactose. Our matchmaker above is great at setting up dates between young men and women, but completely useless in helping dogs find their true love!
- Enzymes are often named for the substrates on which they act. Thus the enzymes involved in the reactions above would be sucrase and lactase respectively. Notice that the suffix –ase is added to the name of the substrate.
- An enzyme’s shape governs its function. Each enzyme has an active site where only certain molecules (substrates) can bind. When the substrate(s) bind to the active site the enzyme catalyzes the chemical reaction and then they are released as a new product.
- Enzymes are sensitive to changes in temperature and pH. One way to speed up chemical reactions is to turn up the heat, but increasing temperature too much can alter or even destroy cells. Enzymes in the human body function optimally between 35-40° C (95-104° F). They also function best at around neutral pH, with a range typically between 6-8. If we change the temperature or pH to values outside the optimum, the enzymes may change shape and lose their function.
- Enzymes may require “helper” substances to catalyze chemical reactions. These helpers are termed cofactors or coenzymes. Cofactors are inorganic substances such as zinc or iron. Coenzymes are organic molecules like vitamins.
**You may use the buttons below to go to the next or previous reading in this Module**

