ORGANIC CHEMISTRY
CARBOHYDRATES
There are roughly 92 naturally occurring elements on earth, but interestingly, only 4 (oxygen, carbon, hydrogen and nitrogen) make about 96% of the mass of the human body. These elements combine to form life-sustaining biomolecules, which can be divided into four groups: carbohydrates, lipids, proteins, and nucleic acids. Carbohydrates, proteins, and lipids are used by cells as the building blocks for cells or for energy, while nucleic acids are the basis of the genetic material. Carbohydrates are the most abundant of the biomolecules. Each year the earth converts more than 100 billion metric tons of CO2 and H2O into carbohydrates. If we were to identify the most important carbohydrate molecule on the planet, in terms of its ability to sustain life, we would undoubtedly select the monosaccharide glucose. Without glucose, nearly all animal life as we know it could not exist.
There are three major classes of carbohydrates; monosaccharides, disaccharides, and polysaccharides. This classification is based on how many "subunits make up the molecule. The name "saccharide" is derived from the Greek, meaning sugar. Monosaccharides are the simplest form of carbohydrates and are composed of a single molecule or subunit. The disaccharides are composed of two monosaccharides linked together, and polysaccharides are composed of 3 or more monosaccharides linked together. We will now examine each of these types of carbohydrates.
MONOSACCHARIDES
The monosaccharides (mono = one, saccharide = sugar) are the basic subunits of carbohydrates. They contain from 3 to 7 carbons and have the general formula of (CH2O)n where n ranges from 3 to 7 (5 or 6 being the most common). For example, if n = 6, the formula for the monosaccharide would be C6H12O6 and if n = 5 the formula would be C5H10O5. Hopefully, it is obvious that the monosaccharides contain a significant amount of oxygen, one for every carbon in the molecule. Carbohydrates have the highest oxygen to carbon ratio of any of the important organic molecules. Common monosaccharides include: glucose, fructose, galactose, ribose, and deoxyribose. Notice that the name of each of these sugars ends with the suffix -ose. This suffix, -ose, means full, specifically, full of oxygen. The names of most all sugars will end with this suffix.
The structures of three common monosaccharides are shown in the figure below. Note that the molecules can exist in two different forms. When they are in a dry or powdered state, they exist as a linear molecule (top), but when dissolved in water they adopt a ringed form with oxygen being one of the members of the ring (bottom). Since all of the molecules in our bodies exist as aqueous solutions, the ringed form is how we find monosaccharides in the body. Note also that all three of these compounds have 6 carbons, hence, they have the same molecular formula, C6H12O6. However, their structural formulas are different (see figure below). Molecules that have the same molecular formula but different structural formulas are known as isomers. Even though they each have 6 carbons, 12 hydrogens, and 6 oxygens, they have very different biological actions because of their different structural forms. For example, there are specific carriers that can bring glucose into the cell but do not transport fructose.
Glucose, also called dextrose, is the predominant sugar in our blood. When we speak of blood sugar levels we are really talking about blood glucose levels. We get glucose primarily from the digestion of disaccharides and polysaccharides. Once these carbohydrates are broken down to glucose in the small intestine the glucose is absorbed into the blood and transported to the various organs of the body. There, it can be metabolized by the tissues to provide fuel for cellular metabolism, or, if it is not immediately needed for metabolism, it can be stored as glycogen (more about this complex carbohydrate later) in the liver and muscle or converted to triglycerides (fat) and stored in the fat cells. When the levels of glucose in the blood become low (like they do on fast Sunday), glycogen in the liver can be broken down to release the glucose into the blood, or the body can actually make new glucose molecules from proteins in a process called gluconeogenesis.
Other monosaccharides we need to be aware of are fructose and galactose (6 carbon sugars or hexoses), which are the subunits of the important disaccharides. Also, ribose and deoxyribose (5 carbon sugars or pentoses) which are important components of nucleic acids.
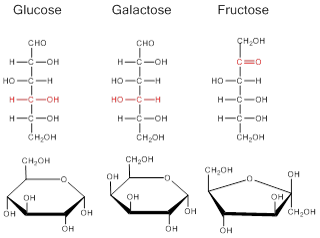
Image created by MG, 2013
The image above shows linear and ring structures of three common monosaccharides. All have the same molecular formula (C6H12O6), but they have different structures (red) and are therefore isomers of each other.
DISACCHARIDES
Disaccharides (Di = 2, saccharide = sugar) are formed when two monosaccharide molecues are linked together. As shown in the figure below, when the two monosaccharides are linked together, one of the products of the reaction is water. Because water is removed to link the subunits together, the reaction is called a dehydration synthesis reaction. This is a common type of synthesis reaction that we will see again when we learn about the formation of lipids and proteins.

Image created by MG, 2013
Dehydration synthesis reaction combines two monosaccharides (glucose) to create a disaccharide (maltose).
There are three important disacchrides that we will discuss; sucrose, lactose, and maltose. In all three of these disaccharides, glucose is one of the monosacchrides that make them up. The figure below shows the structure of these disaccharides and Table 1 outlines their characteristics.
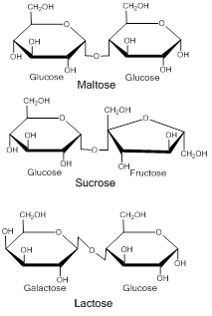
Image created by MG, 2013
The image above shows the structures of the three common disaccharides. All contain glucose as one of their subunits, the difference between the three is the second subunit.
Table 1. Characteristics of three common disaccharides.
|
Name |
Combined Monosaccharides |
Nutritional Information |
|
Sucrose |
Glucose + Fructose |
The most common dietary disaccharide. Naturally found in beet and cane sugar, brown sugar, maple syrup, and honey. You know it as table sugar. |
|
Lactose |
Glucose + Galactose |
Found in dairy products. This is the least sweet of the disaccharides. |
|
Maltose |
Glucose + Glucose |
Found in foods including breakfast cereals, germinating seeds, and beer. |
Only monosaccharides can be absorbed from the digestive tract into the blood, therefore, in order to enter the body, disaccharides must first be digested into their monosaccharide subunits. In the small intestine are specific enzymes for each of these: sucrase to digest sucrose, lactase to digest lactose, and maltase to digest maltose. The reaction for digestion is essentially the reverse of the dehydration synthesis reaction, i.e. water is added back into the bond to break it. This type of reaction is called a hydrolysis reaction. The figure below shows an example of a hydrolysis reaction. Because disaccharides are easily digested and quickly absorbed into the blood, they, along with the monosaccharides, are often referred to as the simple sugars.
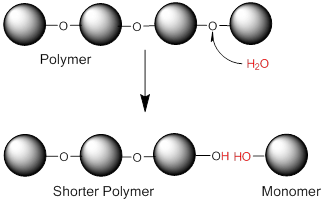
Image created by BYU-I Student Hannah Crowder, 2013
The image above shows a hydrolysis reaction. Bonds between the monomers in a polymer can be broken by the enzymatic addition of water to the bonds.
You may know someone who is lactose intolerant, or you may be lactose intolerant yourself. Most mammals do not consume milk once they are adults and no longer need the enzyme to digest lactose, hence the body stops making the enzyme. If lactose is not broken down into its monosaccharide subunits it cannot be absorbed and passes into the large intestine. The bacteria that live in the large intestine love lactose and start eating it. Unfortunately, when they eat a lot of lactose they produce a lot of gas. Also, the lactose pulls water into the large intestine with it by osmosis. Symptoms of lactose intolerance include abdominal bloating, diarrhea, abdominal cramps, flatulence (gas), and nausea. The symptoms are due to undigested lactose moving into the large intestine. World-wide, about 75% of the adult population experiences some degree of lactose intolerance, however, the incidence differs greatly from country to country (see figure below). Typically, northern Europeans and their descendants have the lowest incidence, mainly due to the fact that in their culture, cattle and goats were domesticated long ago and the milk products from these animals remains an important source of nutrition. Although not depicted on the maps, the Masai tribesmen of Eastern Africa also exhibit a low incidence of lactose intolerance, also attributed to their tradition of raising cattle and goats for the milk products.
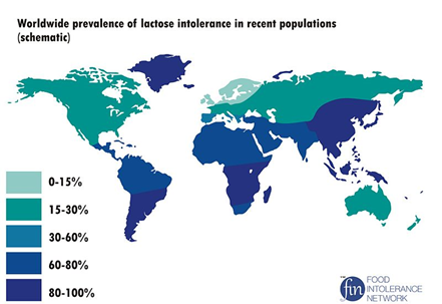
Worldwide incidence of lactose intolerance. Image downloaded from Wikimedia Commons Dec, 2013: Author: NmiPortal; Site: https://commons.wikimedia.org/wiki/File:Worldwide_prevalence_of_lactose_intolerance_in_recent_populations.jpg; License:Creative Commons Attribution-Share Alike 3.0 Unported
POLYSACCHARIDES
Polysaccharides are long chains of monosaccharide subunits linked together through dehydration synthesis reactions. These chains may number from as few as three subunits to thousands. The polysaccharides are what we refer to as complex carbohydrates. Based on their function, polysaccharides can be classified as either storage molecules, or structural molecules. Storage polysaccharides include starch and glucogen. Starch is a large polymer of glucose subunits and is the storage form of glucose in plants. Sources include seeds, grains, corn, beans, potatoes, and rice.
There are actually two types of starch: amylose and amylopectin. Amylose is a long, unbranched, chain of glucose subunits. Amylopectin, on the other hand has a branched structure (see figure below). It is the proportion of each form of starch in a particular food that determines the food's ability to be digested. Foods with a large amount of amylopectin are digested and absorb rapidly, while foods that have higher levels of amylose break down at a slower rate.
Glycogen is the storage form of carbohydrate in animals. Glycogen, like starch, is a polymer of glucose subunits. It is similar in structure to amylopectin but it is even more highly branched. We store glycogen primarily in our livers and skeletal muscles. The glycogen in skeletal muscle can be depleted with as little as 1 hour of vigorus exercise. On the other hand, during a fast, liver glycogen will last 12-24 hours. That shaky feeling you get at the end of your fast on Fast Sunday is largely due to a depletion of your glycogen stores.
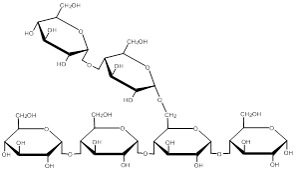
Image created by MG, 2013
The image above shows branching in a polysaccharide molecule.
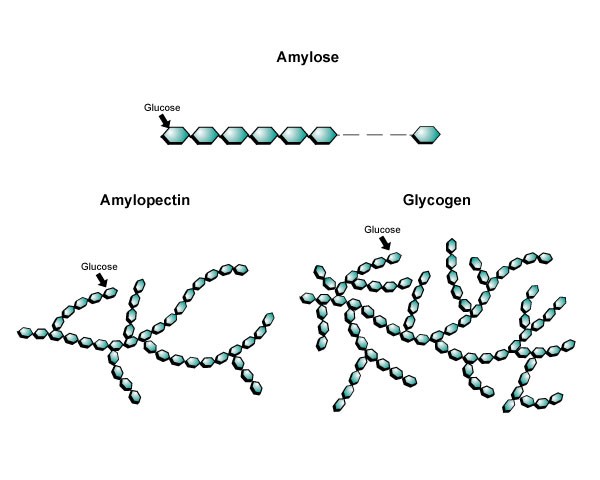
Image created by BYU-I student Hannah Crowder, 2013
This image above shows different degrees of branching in Amylose, Amylopectin, and Glycogen.
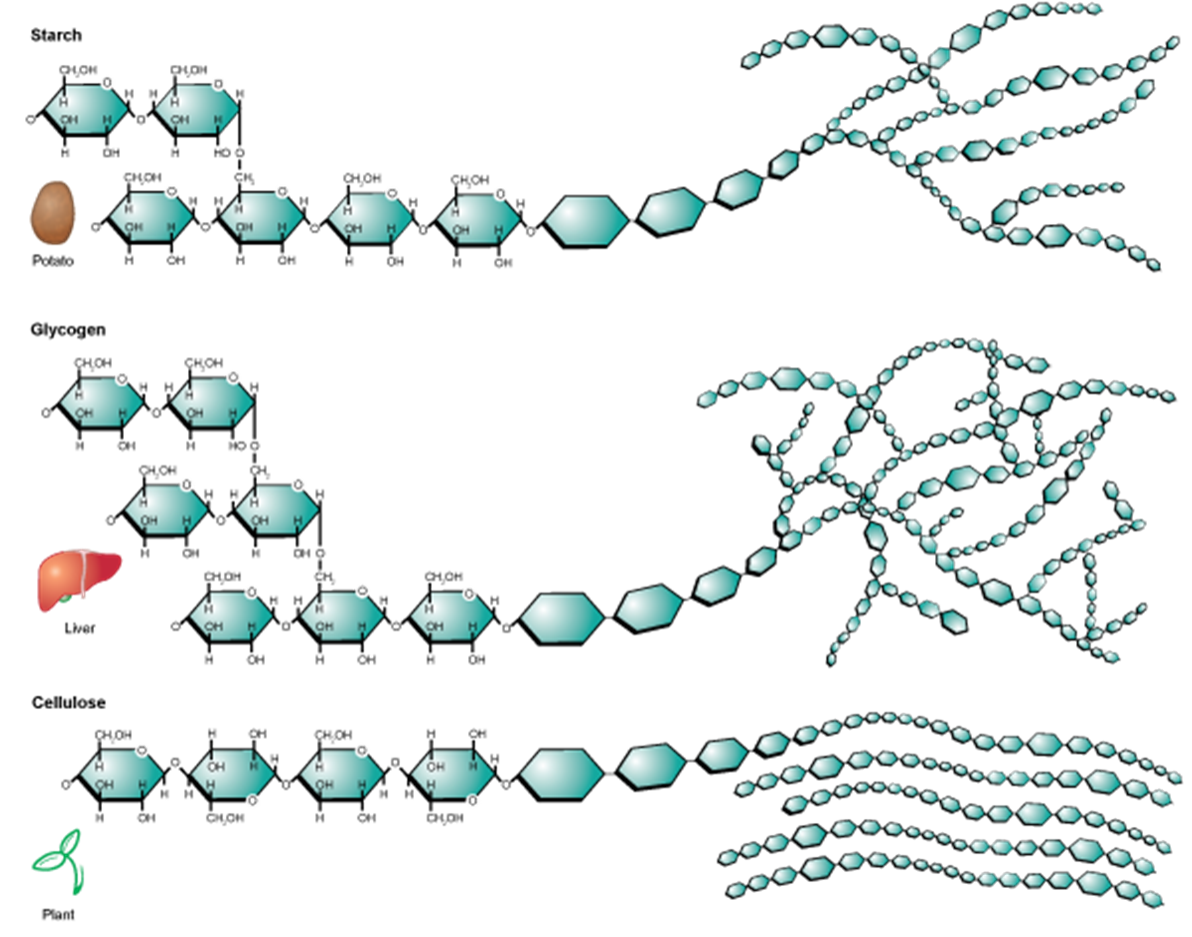
Image created by BYU-I student Hannah Crowder, 2013
Another figure depicting the levels of branching in Starch, Glycogen, and Cellulose.
The branched structure of glycogen allows for easy breakdown by enzymes in the body to release the glucose so that it can be utilized for energy. Glycogen stored in the muscle provides energy required by the muscle for exercise, especially high-intensity and endurance activities. Glycogen stored in the liver is utilized to provide other tissues with energy, such as the neurons in the nervous system.
An important structural polysaccharide is cellulose. Cellulose is an important structural molecule in plants, and provides fiber that we need in our diets. Cellulose is a polymer of glucose. However, unlike starches and glycogen, we do not have the enzymes to digest cellulose. This is due to a difference in the configuration of the bonds between the glucose monomers (see figure below). Cellulose forms the structural components of plant cell walls. It is especially plentiful in leafy vegetables and in whole grains. Although we cannot digest cellulose for energy, it provides bulk to the stool and may reduce the risk of some diseases like diverticular disease and colon cancer.
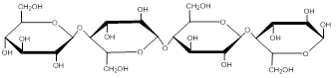
Image created by MG, 2013
The image above shows bonding of glucose monomers in cellulose. Note that the configuration is different than in starch and glycogen (see figure above). We do not have enzymes to digest the bonds in cellulose.
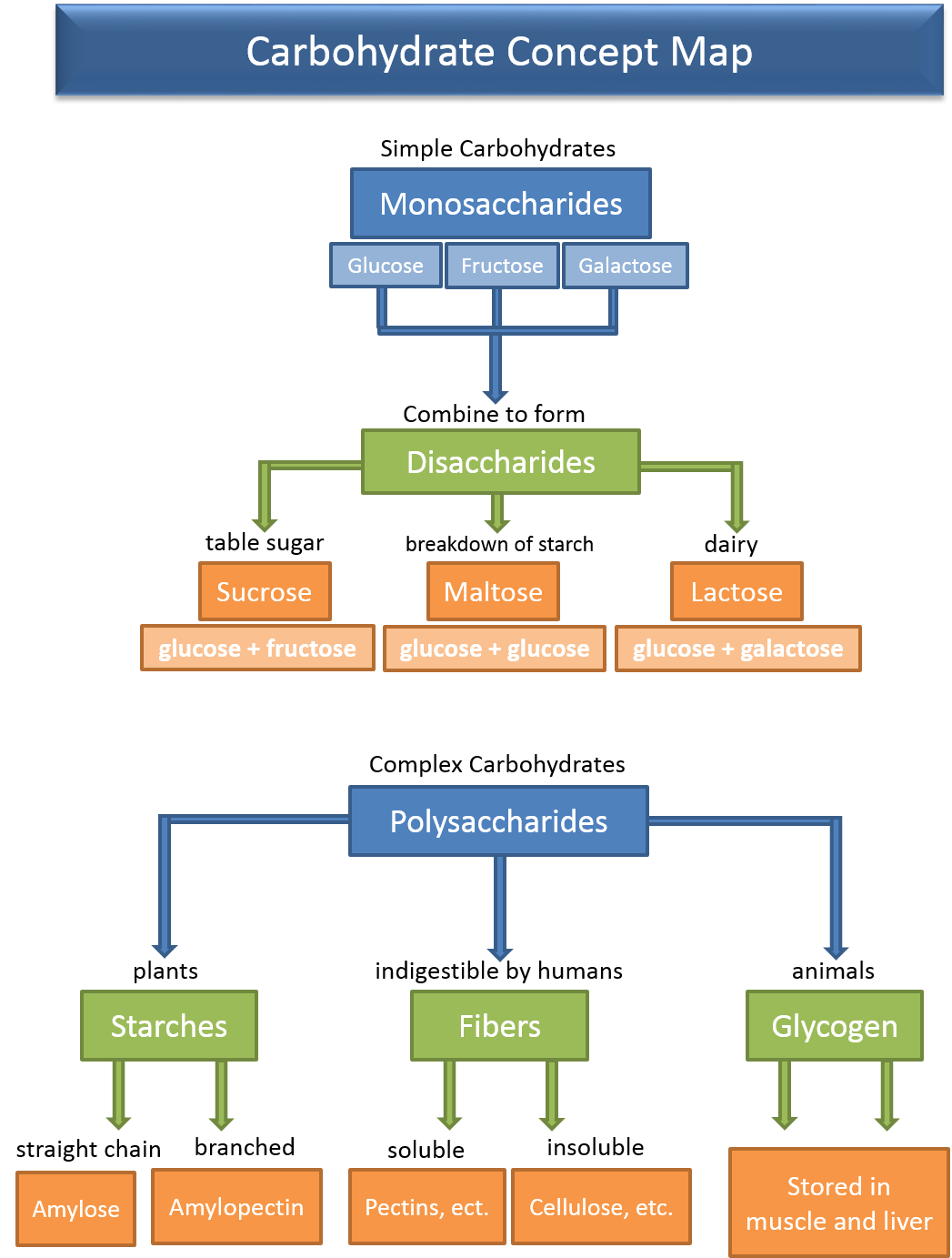
Image created by BYU-I student Hannah Crowder, 2013
HEALTH NOTE
It is safe to say that carbohydrates are an important part of a healthy diet, although some carbohydrates are better than others. When we consume simple sugars, they are quickly absorbed and blood sugar levels rise rapidly. This, in turn, results in secretion of large amounts of insulin followed by a rapid drop in blood sugar. This is probably not ideal. Indeed, a recent study1 reported that consuming just one sugary soft drink per day increased the risk of developing coronary heart disease by 20% in men. Consumption of sugar laden soft drinks has also been shown to increase the incidence of obesity which increases the risk of type 2 diabetes. Complex carbohydrates found in whole grains, on the other hand, tend to offer positive health benefits.
One current topic of intense interest is the question of high-fructose corn syrup. High-fructose corn syrup is produced from corn starch which is a polymer of glucose. The starch is hydrolyzed to separate the glucose monomers and then chemically treated to convert some of the glucose to fructose. Most high-fructose corn syrup is 55% fructose and 45% glucose. Fructose is handled by the body differently than glucose. Whereas glucose can enter nearly all cells of the body (some cells need a little help from insulin to take up glucose), fructose is metabolized almost exclusively by the liver. There seems to be mounting evidence that high-fructose corn syrup may be bad for us. In a recent study in rats comparing high-fructose corn syrup with sucrose, rats consuming high-fructose corn syrup had greater weight gain, increased amounts of visceral fat (the fat around our abdominal organs), and an increase in the levels of circulating triglycerides2 (triglycerides are the main component of the fat in our adipose cells). Although there are those that still argue that high-fructose corn syrup is no worse for you than sucrose, the growing body of evidence seems to suggest differently. So, next time you sit down with a nice cold glass of Sprite, think about what you might be doing to your body.
References
1. Koning, L. de, et al. Sweetened Beverfage Consumption, Incident of Coronary Heart Disease and Biomarkers of Risk in Men. Circulation (on-line) Mar 12, 2012
3. Bocarsly, M.E. et al. High Fructose Corn Syrup Causes Characteristics of Obesity in Rats: Increased Body Weight, Body Fate, and Triglyceride Levels. Pharmacology, Biochemistry and Behavior. 97:101-106-2012
**You may use the buttons below to go to the next or previous reading in this Module**

