METABOLISM
ENERGY CYCLE
"There is no easy way out. If there were, I would have bought it. And believe me, it would be one of my favorite things."
-Oprah Winfrey
Have you ever noticed that in our world there seems to be a never ending parade of "fads?" This is especially true with diet and exercise. Such "fads" promise quick weight loss, instant body shaping or both? Weeding out false information and gimmicks can be more difficult than it may seem. There are so many testimonials, "evidence," and promises that clever advertisers can leave you feeling that you are the only one not taking advantage of a "sure thing." Ultimately, it is just too tempting for many and soon we are shelling out hard earned dollars for something that is here today, gone tomorrow and we are little changed from the experience. Weight loss and fitness gimmicks make up a billion dollar industry which leaves us with an awful lot of pills, powders and plans to sort through.
A few years ago, I found myself fascinated by an article in Time magazine.
(http://www.time.com/time/health/article/0,8599,1890175,00.html)
This article suggests that a type of fat exists that can make us thin. It is called "brown fat." This article and others like it claim that the activation of Brown Fat would cause weight loss with no need for diet or exercise. Evidence that this might work comes from research that shows mice getting skinny when brown fat is activated in their bodies. Also, it has been shown that many humans who maintain a lean physique have higher quantities of Brown Fat. Researchers tell us that as little as 50g of brown fat can consume as much as 20% of the total daily caloric expenditure (Lidell and Enerback, 2010). However, it is not known how to activate, regulate or increase brown fat in humans. It is also unknown what kinds of side effects this might cause if it was attempted.
You must have some questions by now. Wouldn't you like to know what brown fat is? How does it work? Does it work? Why don't we hear about medical treatments that use it? How does brown fat burn energy instead of store it? What makes fat brown instead of white?
You know that the media has an insatiable appetite to advertise the next "greatest thing" with regard to diet and exercise. Your ability to react intelligently to this type of information will be greatly enhanced through a solid understanding of Metabolism.
Metabolism in its most simplified "big picture" description is largely a story of chemical bonds, more specifically the electrons in these bonds. You probably recall that electrons are a subatomic particle with a negative charge. Electrons also move with considerable energy and speed as they find themselves drawn to protons (which are positive and found in atomic nuclei). So, what if we could take some high energy electrons and store them somewhere. Later, when we wanted to harvest some of the electron energy to do some work we could go get them. This is kind of how a battery works. Smart people have figured out how to store high energy electrons and when we want these electrons to do something (like flow through a flashlight filament) we can get them and use them. Living things also use high energy electrons to power the processes of biology. We as humans don't plug ourselves into a Duracell battery, but we do eat foods that have stored high energy electrons.
Examine figure 1. Our story starts with the sun. The earth gets energy from the sun. This energy is called solar energy. You can feel some of this energy when you go out on a warm day. Solar energy is absorbed by plants and when a plant gets solar energy it uses this energy to excite electrons.
An electron in a bond between oxygen and hydrogen has a certain amount of energy. An electron in a bond between carbon and hydrogen has even a greater amount of energy. When sunlight strikes a plant, the solar energy is used to break an oxygen-hydrogen bond and then create a carbon-hydrogen bond. In this very incredible and complex process called photosynthesis, plants use water as the source of oxygen-hydrogen bonds and carbon dioxide (CO2) serves as the source of carbon that will accept a hydrogen with its newly energized electron. Recall that whenever an atom receives more electrons, we say that the atom has been reduced, and whenever an atom loses electrons, we say that the atom has been oxidized. In our story of photosynthesis, CO2 is reduced and H2O is oxidized.
Let's summarize to this point. Plants take CO2 and H2O, they use solar energy to excite some electrons in H2O and then transfer the excited electron with a hydrogen to carbon. Now, we have a higher energy C-H bond. Reduced CO2 can be transformed into a variety of molecules with different numbers of C-H bonds. These molecules make up the sugars, lipids and proteins that you have learned about. If you were to look up the chemical structures of any sugars, lipids, or proteins, you would see a lot of C-H bonds.
So, in a way, plants create a kind of "battery." The carbon-hydrogen bonds formed by plants can exist for a long time as sugars, lipids and proteins. If the high energy electrons are allowed to go back and form an O-H bond, energy would have to be released. This is what our body does. Our cells have the ability to facilitate a transfer of high energy electrons in C-H bonds back to O-H bonds and use the energy that is released.
Now, you hopefully see this cycle of energy. We eat C-H bonds when we consume carbohydrates, lipids and proteins. In our cells we "process" the C-H bonds in a way that allows us to put the high energy electrons back onto Oxygen. Energy is released and we use the energy to run the cells of our body. Also, if you note in figure 1, CO2 and H2O are created again when we allow high energy electrons to return back to oxygen. Plants use the CO2, and the H2O and the cycle continues.
Bioenergetics is the study of how energy is transferred through the chemical reactions of living systems. Our cells are always breaking and making bonds. Keep in mind that whenever we see a reaction that involves the synthesis of new molecules, we call this an Anabolic reaction (think "anabolic steroids" and it shouldn't surprise you that these steroids stimulate reactions that make new proteins in muscle cells).
Catabolic reactions occur when molecules are broken down into smaller and smaller parts. Several catabolic reactions occur in our cells to break sugars, proteins, and lipids down into smaller and smaller parts until finally the C-H bonds have been processed to allow the energy in such bonds to be used. Metabolism refers to all of the catabolic and anabolic processes that a cell is engaged in. The details of metabolism can be daunting and normally require classes in biology and chemistry to understand fully. In this class, we hope to provide a simplified overview of metabolism and show you the "big picture" of how our cells capture energy inherent to electrons in carbon-hydrogen bonds and then use that energy to make ATP.
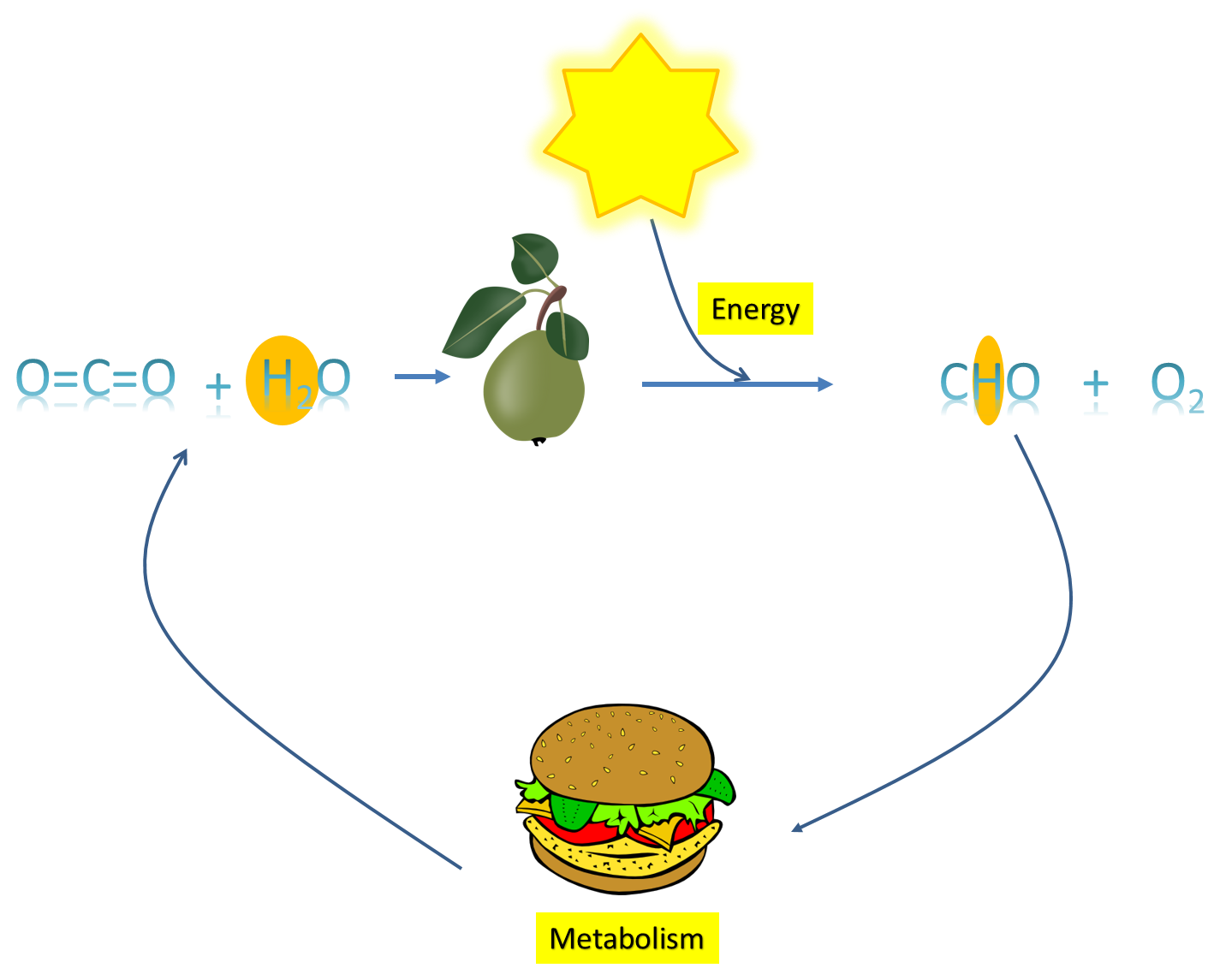
Image created by JS at BYU Idaho. Clipart from clker.com; License Public Domain; http://www.clker.com/clipart-4079.html and http://www.clker.com/clipart-stew-pear-with-leafs.html
The image is a graphical representation of the energy cycle. Solar energy from the sun excites electrons enough that they can facilitate a bond with carbon instead of oxygen (photosynthesis). We eat these C-H bonds in carbohydrates, lipids and proteins. Our body cells allow the high energy electrons in C-H bonds to return to a lower energy state in O-H bonds and we produce CO2 and H2O, which can cycle back to be used in photosynthesis again.
**You may use the buttons below to go to the next or previous reading in this Module**

