METABOLISM
ATP, NAD AND FAD
ATP
Cells use a molecule called Adenosine Triphosphate (or ATP) as an energy source (See figure 2). The phosphates in this molecule can supply energy to substrates in our cells. Enzymes exist in our cells that can remove a phosphate from ATP and attach it to a different molecule-usually a protein (See Figure 3). When this happens, we say that the protein has been phosphorylated. Think of the third phosphate as being a little sack of energy. When it is transferred to a protein, this energy can be used to do something. For example, in figure 3, the protein changes its shape when it becomes phosphorylated. When proteins change their shape, we often call this a conformational change to the protein structure. There are many proteins in the body that use a phosphate from ATP to induce a conformational change. This shifting of the protein shape ultimately allows for things like muscle contraction, cell mobility, membrane transport, and enzyme action. Cells and life exist only if a consistent and steady supply of ATP is available.
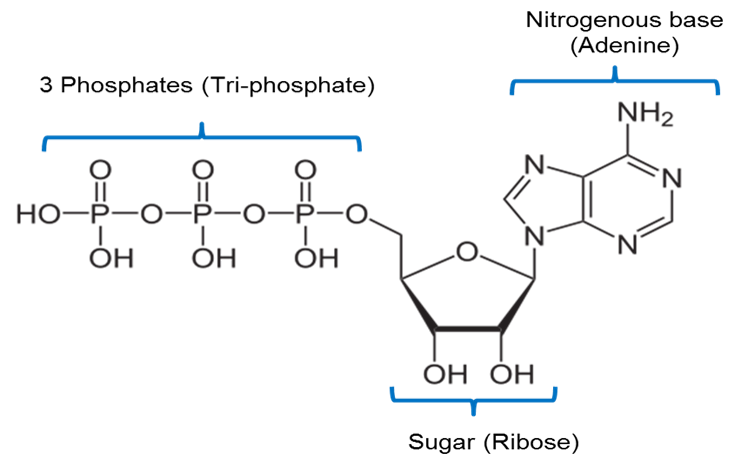
Image created by JS at BYU Idaho F2013.
The image above is a representation of the chemical structure of ATP. ATP includes a nitrogenous base called adenine joined to a 5 carbon sugar called ribose and 3 phosphate groups.
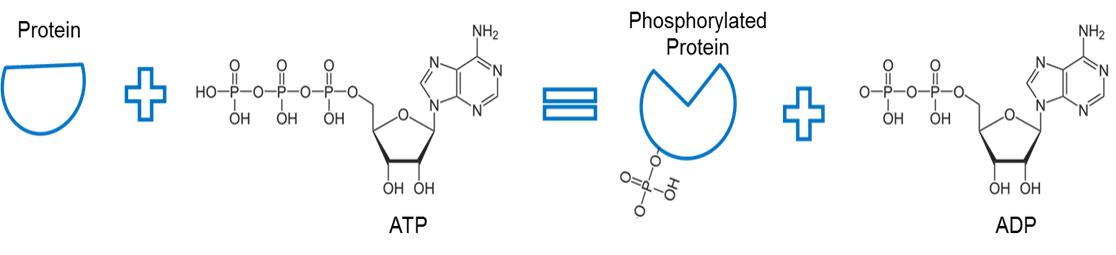
Image created by JS at BYU Idaho F2013.
ATP is used to phosphorylate a protein. An enzyme, called a kinase (not shown) removes a phosphate from ATP and facilitates a bond between the phosphate and some other protein. The bonding of a phosphate to a protein in this manner is called phosphorylation. The phosphate bone with the protein has higher energy. Notice that phosphorylation uses this energy to cause a conformational change of the protein shape.
NAD and FAD
Nicotinamide Adenine Dinucleotide (NAD) and Flavin Adenine Dinucleotide (FAD) are coenzymes involved in reversible oxidation and reduction reactions. It is often stated that these compounds are electron carriers because they accept electrons (become reduced) during catabolic steps in the breakdown of organic molecules such as carbohydrates and lipids. Then, these reduced coenzymes can donate these electrons to some other biochemical reaction normally involved in a process that is anabolic (like the synthesis of ATP).
NAD+ / NADH
Nicotinamide Adenine Dinucleotide in its oxidized state is called NAD+, after being reduced (or accepting electrons), it is referred to as NADH. See figure 4 for a molecular illustration. The vitamin Niacin (also called B3) is used to derive this compound. Niacin provides the organic ring structure that will directly participate in the transfer of a hydrogen atom and 2 electrons. NAD+ is often found in conjunction with a "dehydrogenase" enzyme. A dehydrogenase reaction removes two hydrogen atoms; one as a hydride (:H-) (a hydride is a hydrogen atom with 2 electrons) and one as a hydrogen cation (H+) (and of course, a hydrogen cation has no electrons). The hydride bonds with NAD+ and creates a reduced compound of Nictinamide Adenine Dinucleotide (NADH). The second hydrogen atom (H+) is released into solution see figure 4.
As you examine the reactions for metabolism, look for reactions that yield NADH. NADH will be important as it will deliver the hydrogens and electrons that it picks up to biochemical processes that can use the electrons and hydrogens to make ATP.
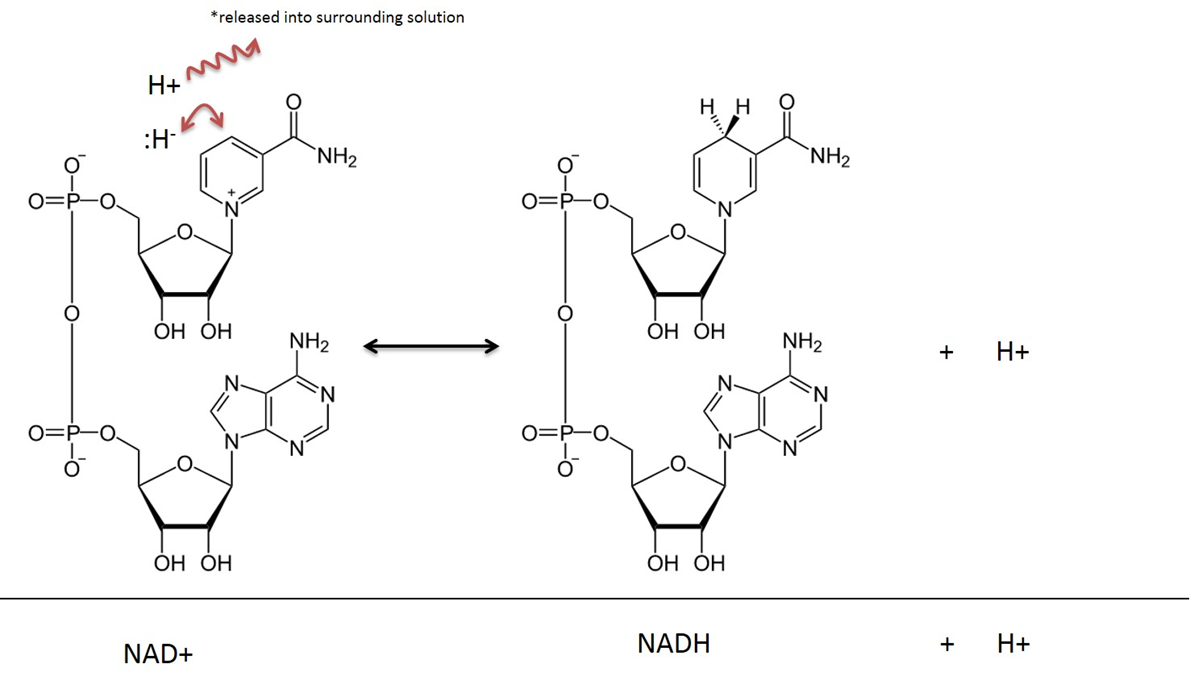
Image created by JS at BYU Idaho F2013.
In metabolic reactions that involve NAD, two hydrogen atoms and two electrons are removed from a substrate and transferred to NAD+. NAD+ accepts a hydride ion (a hydrogen with 2 electrons) and becomes Nicotinamide Adenine Dinucleotide in the reduced form (NADH). The hydrogen cation that is also captured in the reaction is released into the surrounding solution. Remember that this reaction is reversible.
In the explanation of reactions that occur in Metabolism, it is common to ignore the H+ released into solution and this text will depict the outcome of NAD reduction as simply NADH, rather than NADH + H+.
FAD / FADH2
Flavin adenine dinucleotide in its oxidized state is called FAD. After being reduced, it is called FADH2. See figure 5 for a molecular illustration. The vitamin, riboflavin (or B2) is used to derive this compound. Riboflavin provides the ring structures that will directly participate in the transfer of two hydrogen atoms (each with one electron this time). Similar to NAD, FAD works in association with a "dehydrogenase" enzyme. The reaction removes two hydrogen atoms; each a proton with one electron. Both hydrogen atoms bond with FAD. This reaction does not release an H+ into solution like the reduction of NAD does.
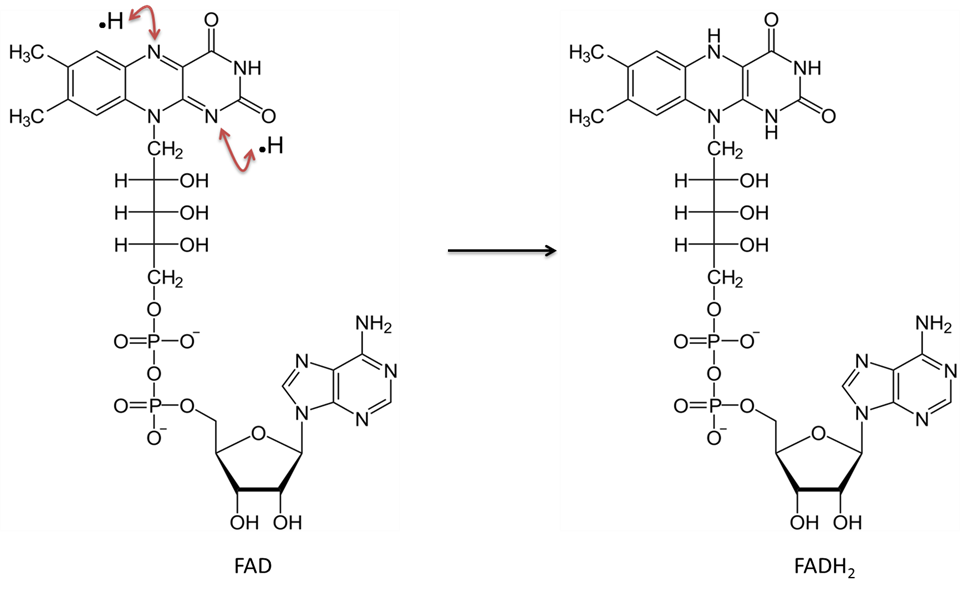
Image created by JS at BYU Idaho F2013.
Flavin adenine dinucleotide in the oxidized form (FAD) accepts two hydrogen atoms (each with one electron) and becomes FADH2.
As you examine the reactions for metabolism, look for a reaction that yields FADH2. Similar to NADH, FADH2 will be important as it will deliver hydrogens and electrons to biochemical processes that can use the electrons and hydrogens to make ATP.
**You may use the buttons below to go to the next or previous reading in this Module**


