MUSCLES-STRUCTURE AND FUNCTION
SKELETAL MUSCLE ORGANIZATION
Before completing this reading, you should complete the following:
Step 01
Download a page sized image of "Muscle Organization.
Step 02
Work through a tutorial that will help you label your image.
Step 03
With your newly labeled image in hand, read through the following paragraphs.
For those who cannot participate in the steps above a Skeletal Muscle Organization Alternative Assignment has been provided, giving you all of the information found in the interactive tutorial. NOTE: This alternative assignment has been temporarily deactivated.
Skeletal Muscle Cells-Gross and Microscopic Structure
Each skeletal muscle cell, also called a muscle fiber, develops as many embryonic myocytes fused into one long, multi-nucleated skeletal muscle cell. These muscle fibers are bound together into bundles, or fascicles, and are supplied with a rich network of blood vessels and nerves. The fascicles are then bundled together to form the intact muscle. Let's dissect a skeletal muscle, beginning with the muscle as a whole externally, and continuing internally down to the submicroscopic level of a single muscle cell.
In an intact skeletal muscle, a rich network of nerves and blood vessels nourish and control each muscle cell. These muscle fibers are individually wrapped and then bound together by several different layers of fibrous connective tissue.
The epimysium ("epi"-outside, and "mysium"-muscle) is a layer of dense fibrous connective tissue that surrounds the entire muscle. This layer is also often referred to as the fascia. Each skeletal muscle is formed from several bundled fascicles of skeletal muscle fibers, and each fascicle is surrounded by perimysium ("peri"-around). Each single muscle cell is wrapped individually with a fine layer of loose (areolar) connective tissue called endomysium ("endo"-inside). These connective tissue layers are continuous with each other and they all extend beyond the ends of the muscle fibers themselves, forming the tendons that anchor muscles to bone, moving the bones when the muscles contract.
Deep to the endomysium, each skeletal muscle cell is surrounded by a cell membrane known as the sarcolemma (You will see the prefixes sarc- and myo-quite a bit in this discussion, so you should understand that these are prefixes that refer to "muscle"). The cytoplasm, or sarcoplasm contains a large amount of glycogen (the storage form of glucose) for energy, and myoglobin-a red pigment similar to hemoglobin that can store oxygen. Most of the intracellular space, however, is taken up by rod-like myofibrils-cylindrical protein structures. Each muscle fiber contains hundreds or even thousands of myofibrils that extend from one end of each muscle fiber to the other. These myofibrils take up about 80% of the intracellular space, and are so densely packed inside these cells that mitochondria and other organelles get sandwiched between them while the nuclei get pushed to the outside and are located on the periphery right under the sarcolemma.
Each myofibril is comprised of several varieties of protein molecules that form the myofilaments, and each myofilament contains the contractile segments that allow contraction. These contractile segments are known as sarcomeres ("sarc-" - muscle, "mere" - part). The striations seen microscopically within skeletal muscle fibers are formed by the regular, organized arrangement of myofilaments-much like what we would see if we painted stripes on chopsticks and bundled them together with plastic wrap, with the plastic representing the sarcolemma.
The striations microscopically visible in skeletal muscle are formed by the regular arrangement of proteins inside the cells. Notice that there are light and dark striations in each cell. The dark areas are called A bands, which is fairly easy to remember because "a" is the second letter in "dark." The light areas are called I bands, and are also easy to remember because "i" is the second letter in "light." ("A" actually stands for anisotropic, and "I" stands for isotropic. Both of these terms refer to the light absorbing character of each band. However, we'll stick to A and I bands.) The image below shows a micrograph of a sarcomere along with a drawing representing the different parts of the sarcomere.
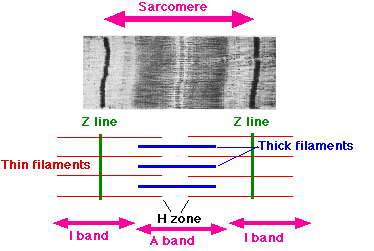
Title: File:Sarcomere.gif; Author: Sameerb; Site: https://commons.wikimedia.org/wiki/File:Sarcomere.gif; License: Public Domain, No restrictions
Notice that in the middle of each I band is a darker line called the z line or z disc. The Z lines are the divisions between the adjacent sarcomeres. Sarcomeres are connected end to end along the entire length of the myofibril. Also, in the middle of each A band is a lighter H zone (H for "helle"-"bright"), and each H zone has a darker M line (M for "middle") running right down the middle of the A band.
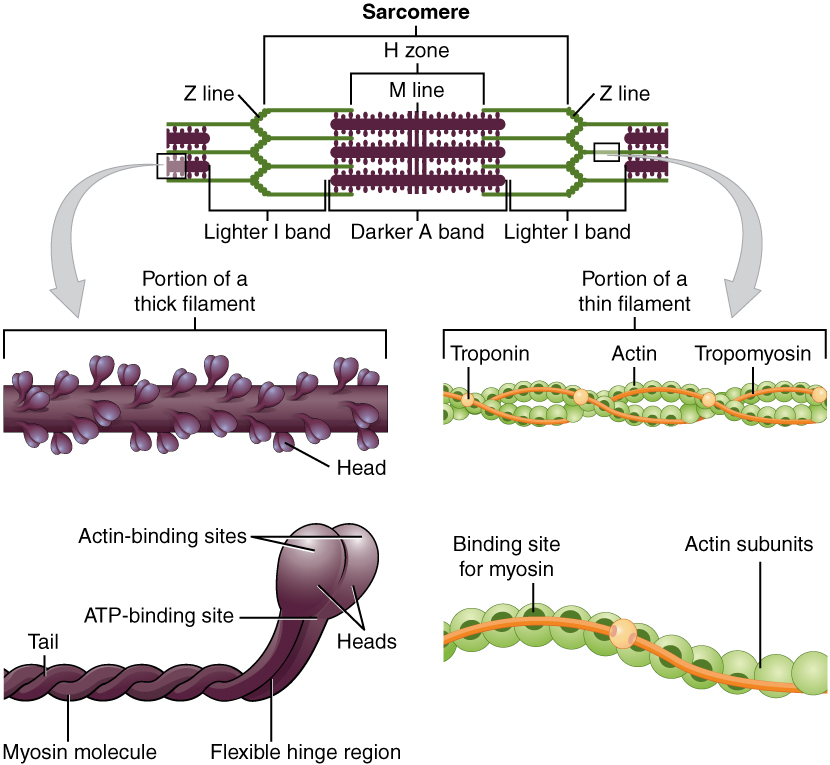
Title: 1003_Thick_and_Thin_Filaments.jpg; Author: OpenStax College; Site: http://cnx.org/contents/6df8aab3-1741-4016-b5a9-ac51b52fade0@3/Skeletal-Muscle; License: licensed under a Creative Commons Attribution 4.0 License.
Each myofibril, in turn, contains several varieties of protein molecules, called myofilaments. The larger, or thick myofilaments are made of the protein, myosin, and the smaller thin myofilaments are chiefly made of the protein, actin.
Let's discuss each myofilament in turn. Each actin molecule is composed of two strands of fibrous actin (F actin) and a series of troponin and tropomyosin molecules. Each F actin is formed by two strings of globular actin (G actin) wound together in a double helical structure, much like twisting two strands of pearls with each other. Each G actin molecule would be represented by a pearl on our hypothetical necklace. Each G actin subunit has a binding site for the myosin head to attach to the actin. Tropomyosin is a long string-like polypeptide that parallels each F actin strand and functions to either hide or expose the "active sites" on each globular actin molecule. Each tropomyosin molecule is long enough to cover the active binding sites on seven G-actin molecules. These proteins run end-to-end the entire length of the F actin. Associated with each tropomyosin molecule is a third polypeptide complex known as troponin. Troponin complexes contain three globular polypeptides (Troponin I, Troponin T, and Troponin C) that have distinct functions. Troponin I binds to actin, Troponin T binds to tropomyosin and helps position it on the F actin strands, and Troponin C binds calcium ions.There is one troponin complex for each tropomyosin. When calcium binds to Troponin C, it causes a conformational change in the entire complex that results in exposure of the myosin binding sites on the G actin subunits. More on this later.
The thick myofilaments are composed chiefly of the protein myosin, and each thick myofilament is composed of about 300 myosin molecules bound together. Each myosin is made up of 6 proteins subunits, 2 heavy chains and 4 light chains. The heavy chains have a shape similar to a golf club, having a long shaft-like structure, to which is connected the globular myosin head. The shafts, or tails wrap around each other and interact with the tails of other myosin molecules, forming the shaft of the thick filament. The globular heads project out at right angles to the shaft. Half of the myosin molecules have their heads oriented toward one end of the thick filament, and the other half are oriented in the opposite direction. It is the myosin heads that bind to the active sites on the actin. The connection between the head and the shaft of the myosin molecules functions as a hinge, and as such is referred to as the hinge region. The hinge region can bend, and as we shall see later, creates the power stroke when the muscle contracts. The center of the thick filaments are composed only of the shaft portions of the heavy chains. Additionally, each myosin head has an ATPase that binds to and hydrolyses ATP during muscle contraction. It is the ATP that provides the energy for muscle contraction. Each of the myosin heads is associated with two myosin light chains that play a role in regulating the actions of the myosin heads, but the exact mechanism is not fully understood. The three dimensional arrangement of the myosin heads is very important. Imagine that you were looking at a thick filament from the end, and that there is a myosin head sticking straight up. As you moved around the circumference of the thick filament, you would see myosin heads every 30 degrees. This allows each thick filament to interact with 6 thin filaments. Likewise, each thin filament can interact with three thick filaments. This arrangement requires that there be two thin filaments for every thick filament in the myofibril (see image below).
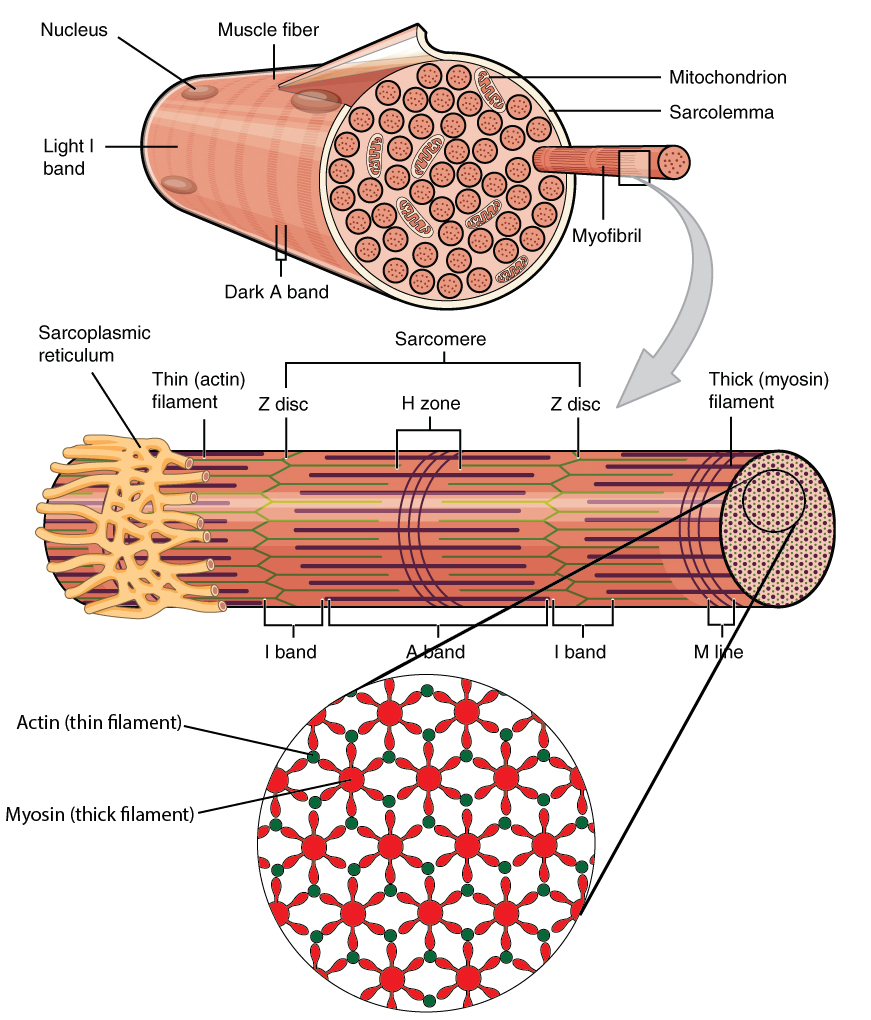
Adapted from the following image:Title: 1022_Muscle_Fibers_(small).jpg; Author: OpenStax College; Site: http://cnx.org/contents/6df8aab3-1741-4016-b5a9-ac51b52fade0@3/Skeletal-Muscle; License: licensed under a Creative Commons Attribution 4.0 License.
During muscle contraction, the myosin heads link the thick and thin myofilaments together, forming cross bridges that cause the thick and thin myofilaments to slide over each other, resulting in shortening of each sarcomere, each skeletal muscle fiber, and the muscle as a whole-much like the two parts of an extension ladder slide over each other. To summarize, in order for the shortening of the muscle to occur, the myosin heads have three important properties: 1. The heads can bind to active sites on G-actin molecules, forming cross bridges. 2. The heads are attached to the rod-like portions of the heavy myosin molecules by a hinge region as already discussed, and 3. The heads have ATPase enzymes that can break down ATP, using the resulting energy to bend the hinge region and allowing detachment of the myosin heads from actin.
The Z-line (or Z-disc) is composed of proteins (alpha actinin) which provide an attachment site for the thin filaments. Likewise, the M-line is composed of proteins (myomesin) that hold myosin molecules in place. The A band is formed by myosin molecules, and the I band is the location where thin filaments do not overlap the thick filaments. The H zone is that portion of the A band where the thick and thin filaments do not overlap.
There is another important structural protein that extends from the Z disc to the M line, running within the thick filament. Due to its large size, this protein is called titin (titin is the largest known protein in the human body and has roughly 30,000 amino acids). It forms the core of the thick myofilaments, holding it in place, and thus keeping the A band organized. In addition, titin has the ability to stretch and recoil and functions to prevent overstretching and damage to the muscle as well as to return it to its normal length when the muscle is stretched beyond its normal resting length. Recall that one of the properties of muscle is its elasticity, titin is the protein responsible for this property.
There are several other important structural proteins, but we will only discuss one more: dystrophin. Dystrophin is a protein located between the sarcolemma and the outermost myofilaments. It links actin to an integral membrane protein, which in turn links the muscle cell to the endomysium of the entire muscle fiber. Genetic mutation of the gene coding for dystrophin is one of the root causes of a class of muscle diseases known collectively as muscular dystrophy (MD). The most common form of MD is Duchene muscular dystrophy (DMD), which is inherted in a "sex-linked" fashion and affects boys. Most DMD patients become wheelchair bound early in life, usually by age 12 or so. Difficulty breathing usually become problematic by age 20, and is often the cause of their sadly premature death.
Sarcoplasmic reticulum and T tubules
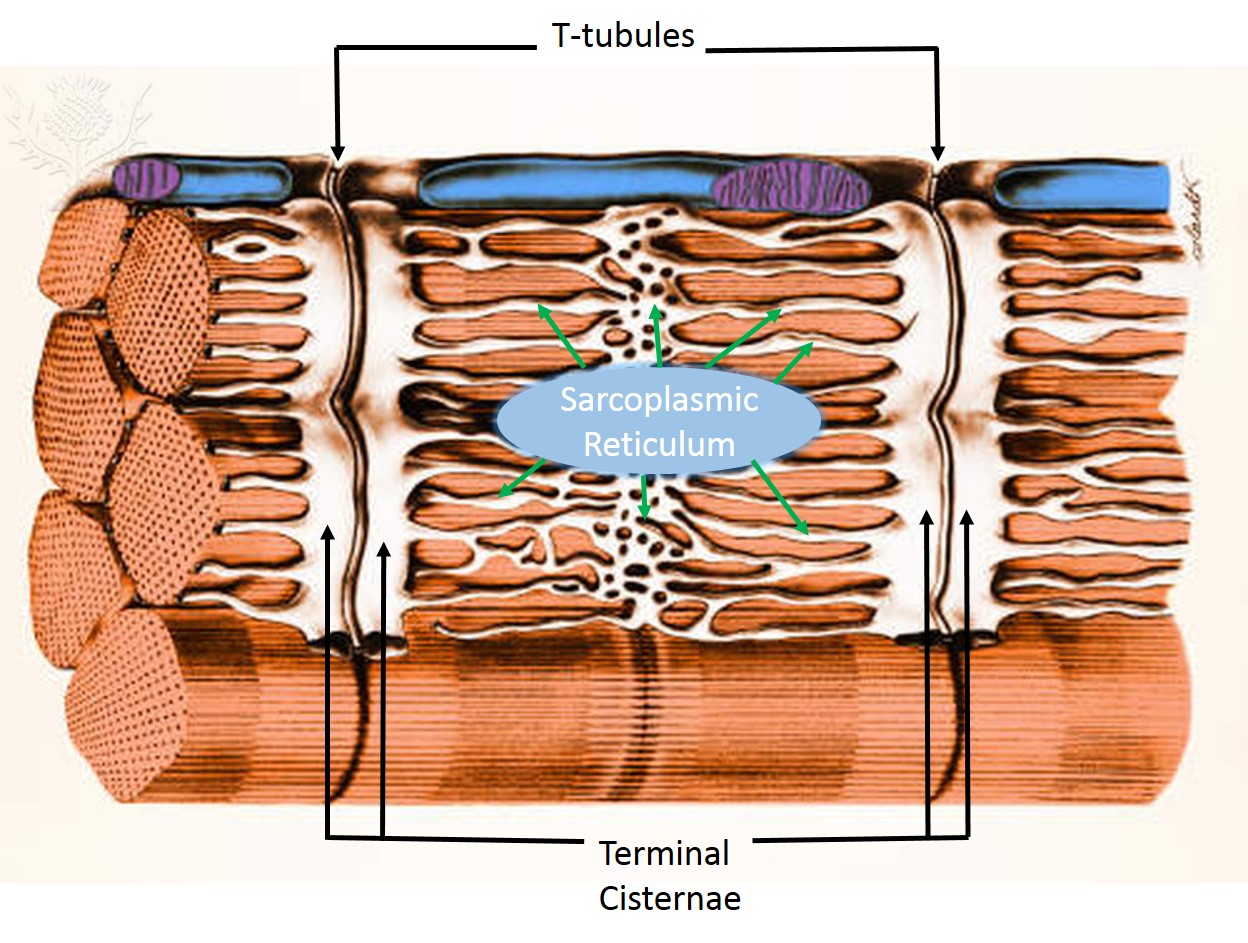
© 2013 Encyclopædia Britannica, Inc. Downloaded and labeled from image quest BYU-Idaho Dec 2013.
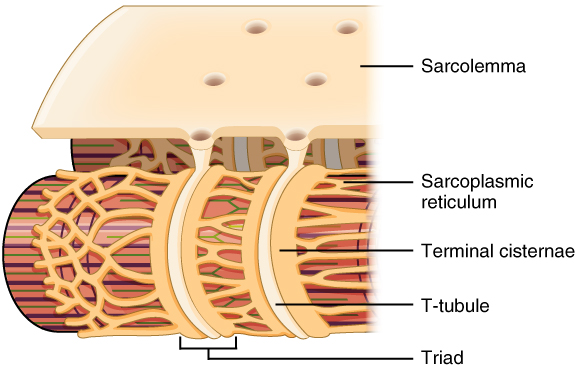
Title: 1023_T-tubule.jpg; Author: OpenStax College; Site: http://cnx.org/contents/6df8aab3-1741-4016-b5a9-ac51b52fade0@3/Skeletal-Muscle; License: licensed under a Creative Commons Attribution 4.0 License.
There are two sets of tubules within skeletal muscles fibers that carry out critical functions during muscle contractions: the sarcoplasmic reticulum and the T tubules.
T tubules (transverse tubules) are invaginations, or indentations of the sarcolemma. They are formed much like a young picky-eater poking holes in his mashed potatoes. T tubules communicate with the extracellular space, and are filled with extracellular fluid. They are located on the sarcomere at the points that the A band and I band overlap. The T tubules are flanked on either side by dilated regions of the cell's endoplasmic reticulum-the sarcoplasmic reticulum. Sarcoplasmic reticulum (SR) is an elaborate network of smooth endoplasmic reticulum that surrounds and encases each myofibril, much like a loosely knitted sweater covers your arms. It stores calcium which can then be released into the sarcoplasm when an action potential is conducted along the sarcolemma of the T tubule. Most of the sarcoplasmic reticulum runs parallel to the myofibrils, but there are right-angle enlargements of the SR at the A band-I band junctions that flank the T tubules. These enlargements are known as terminal cisternae ("end sacs") (see the image above). One T tubule along the two terminal cisternae that parallel it form the triad. The triad is critical in skeletal muscle function. At each triad, the T tubule membrane contains large numbers of voltage-dependant proteins called dihydropyridine (DHP) channels or L-type Calcium channels. Although these are called channels, they do not allow calcium to move through them; rather, they are physically linked to calcium release channels on the terminal cisternae known as Ryanodine receptor channels (RyR). When the membrane is depolarized by an action potential, the DHP channel detects a depolarization and causes the RyR channels to open, resulting in the release of calcium from the terminal cisternae of the SR.
**You may use the buttons below to go to the next or previous reading in this Module**


