Inorganic Chemistry (Atomic Structure)
Electron Configurations
The key factor determining the chemical properties of each element is the configuration of its electrons. Likewise, the energy associated with atoms and molecules is a function of their electrons. Think of the atom; it has a positively charged nucleus with negatively charged electrons orbiting the nucleus. Just like the opposite poles of a magnet, oppositely charged particles attract each other. Because of these attractive forces, it requires energy to pull them apart. In an atom, electrons can be moved further away from the nucleus, but only if energy of some form is applied. Likewise when electrons move closer to the nucleus energy can be released. When I was a kid, we wore fluorescent masks at Halloween. We would shine light on them to “charge” the mask and then when the lights were turned off they would glow or fluoresce. At the time I didn’t know how they worked, I just knew they were fun. Now I know that the light, which is electromagnetic radiation, has energy that can be used to push electrons into orbitals further from the nucleus. When the light is turned off, the electrons “fall” back down into a lower orbital releasing energy, which caused the mask to glow in the dark.
As was mentioned above, the electrons of the atom are located in orbitals. From our discussion above we learned that the energy associated with the electrons in an atom is a function of its position or distance from the nucleus. Therefore, electrons in orbitals close to the nucleus posses less energy than electrons in orbitals that are further away from the nucleus. Another important property of orbitals is that each orbital can hold a maximum of 2 electrons. Based on the amount of energy in each orbital they are arranged into what are referred to as electron shells or energy shells, which contain one or more orbitals. All of the electrons in a given electron shell have the same amount of energy.
To accommodate the electrons in the largest of the naturally occurring elements, seven electron shells are required. However, most biologically important molecules are considerably smaller, so we will only be dealing with the first three energy shells as we discuss electron configuration. The first shell can only accommodate one orbital, thus the maximum number of electrons in the first electron shell is two. The second and third shells each contain four orbitals and can therefore accommodate 8 electrons each. (For those who have or will take more chemistry, I should point out that the third energy shell has more than four orbitals. However, for reasons beyond the scope of this class we can ignore the other orbitals.) One other important fact is that as electrons are added to electron shells, they occupy the innermost shells first before filling the outer shells. It’s like parking spaces at Walmart; those closest to the store fill first, and once they are filled shoppers have no choice but to park in spaces further away. For example, hydrogen has one electron which is located in the first (innermost) electron shell. Helium has two electrons, both in the first energy shell. All of the space in the first energy shell is now filled. Lithium has 3 electrons, two of them are in the first shell and the third electron is in the second electron shell. The reason that this is important to know is because the chemical properties of an element are determined by the number of electrons in its outer electron shell. We define the “outer electron shell” as the last shell that has electrons in it, so for hydrogen, its outer electron shell would be the first shell, and for lithium its outer shell would be the second shell. Each of these elements has one electron in its outer shell, which means that they will have similar chemical properties. So, let’s try an example. Oxygen has an atomic number of 8, which means it has 8 protons and 8 electrons. How many electrons are there in the outer electron shell of oxygen? The first two electrons will go into the first shell leaving 6 to go into the second shell. Therefore, the outer electron shell for oxygen is the second shell and it has 6 electrons in it. See if you can determine the number of electrons in the outer shell for sodium, carbon, potassium, chloride, and neon (see answers at end of chapter).
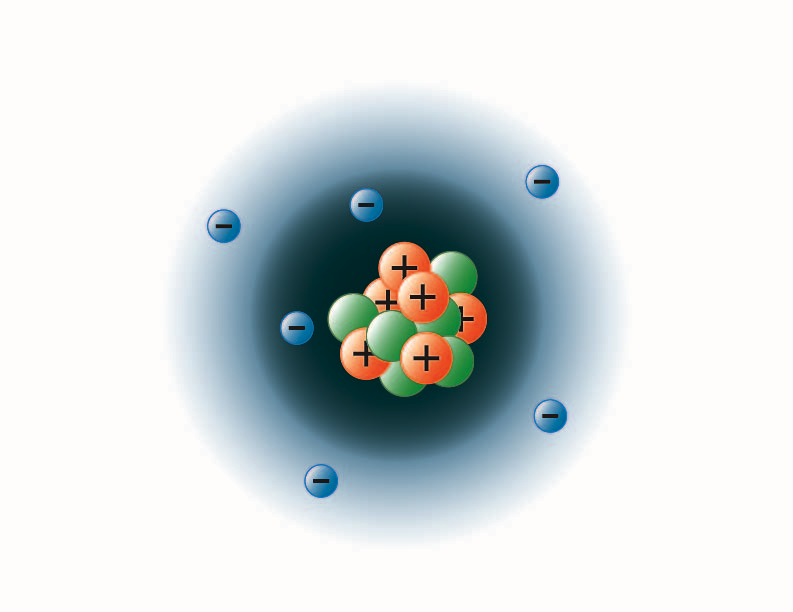
image created by BYU-I student Hannah Crowder Fall 2013
The image above represents the electron configuration for carbon. Carbon has an atomic number of 6, hence 6 electrons. The first two electrons fill the first shell (dark blue) and the next 4 are in the second shell (light blue).
**You may use the buttons below to go to the next or previous reading in this Module**


