Inorganic Chemistry (Atomic Structure)
Chemical Bonds
Close examination of the periodic table will show that the atoms of all of the elements in the last column of the table (i.e. helium, neon, argon, etc) have 8 electrons in their outer shells (with the exception of helium), hence their outer electron shells are full. These elements are all gasses and they are all stable, meaning that they do not react with other elements. Chemists often refer to the “rule of 8” which states that if there are 8 electrons in the outer electron shell, the element is stable. The atoms of all of the other elements have vacancies in their outer electron shells and will react with other atoms to fill their outer shells. The sections below describe some of the important processes by which atoms become stable. The processes that result in the filling of the outer electron shells result in the formation of chemical bonds. In some cases this involves the formation of molecules. Molecules are two or more atoms held together by the sharing of electrons (described below). Molecules composed of more than one type of element can also be called compounds. Hence, H2 (same element) is a molecule, and H2O (different elements) is both a molecule and a compound.
Ionic Bonds
To explain how ionic bonds form we will use common table salt, NaCl, as an example. Sodium has an atomic number of 11, hence, sodium has one electron in its outer electron shell. Chlorine, on the other hand, has an atomic number of 17 and has 7 electrons in its outer shell. When these two elements react, sodium gives the one electron in its outer shell to chlorine. Sodium now has 8 electrons in its outer shell and is stable. However, the result of losing one electron leaves sodium with one more proton than electron and, therefore, it is now an ion with an electrical charge of +1. An ion is an atom that has a net + or – charge. Ions that have a net positive charge are called cations. Chlorine picked up one electron and now its outer electron shell is also full and in the process has become an ion with a -1 charge (one more electron than protons). Ions that have a net negative charge are called anions (think of the term anion as an acronym standing for a negative ion). The opposite charges on these ions create an attraction that will hold them together. We refer to this attraction as an ionic bond. Figure 3 shows the formation of sodium and chloride ions. By changing the electron configuration around these two elements, their chemical properties have been drastically changed. We require NaCl (Na+ and Cl-) for proper function of our bodies but both sodium and chlorine with different electron configurations can be lethal. Chlorine gas, Cl2, is a deadly poison and elemental sodium (no charge) is a metal that ignites when placed in water. This emphasizes the significance of the statement above that the chemical properties of an element are determined by its electron configuration.
It is also important to note that ionic bonds do not form distinct one-to-one attractions between ions, so technically ionic bonds do not form molecules. Instead they form crystalline structures in which each anion is attracted to all of the cations near it and each cation is attracted to all of the anions near it. Even so, you may read or hear NaCl being referred to as a molecule/compound.
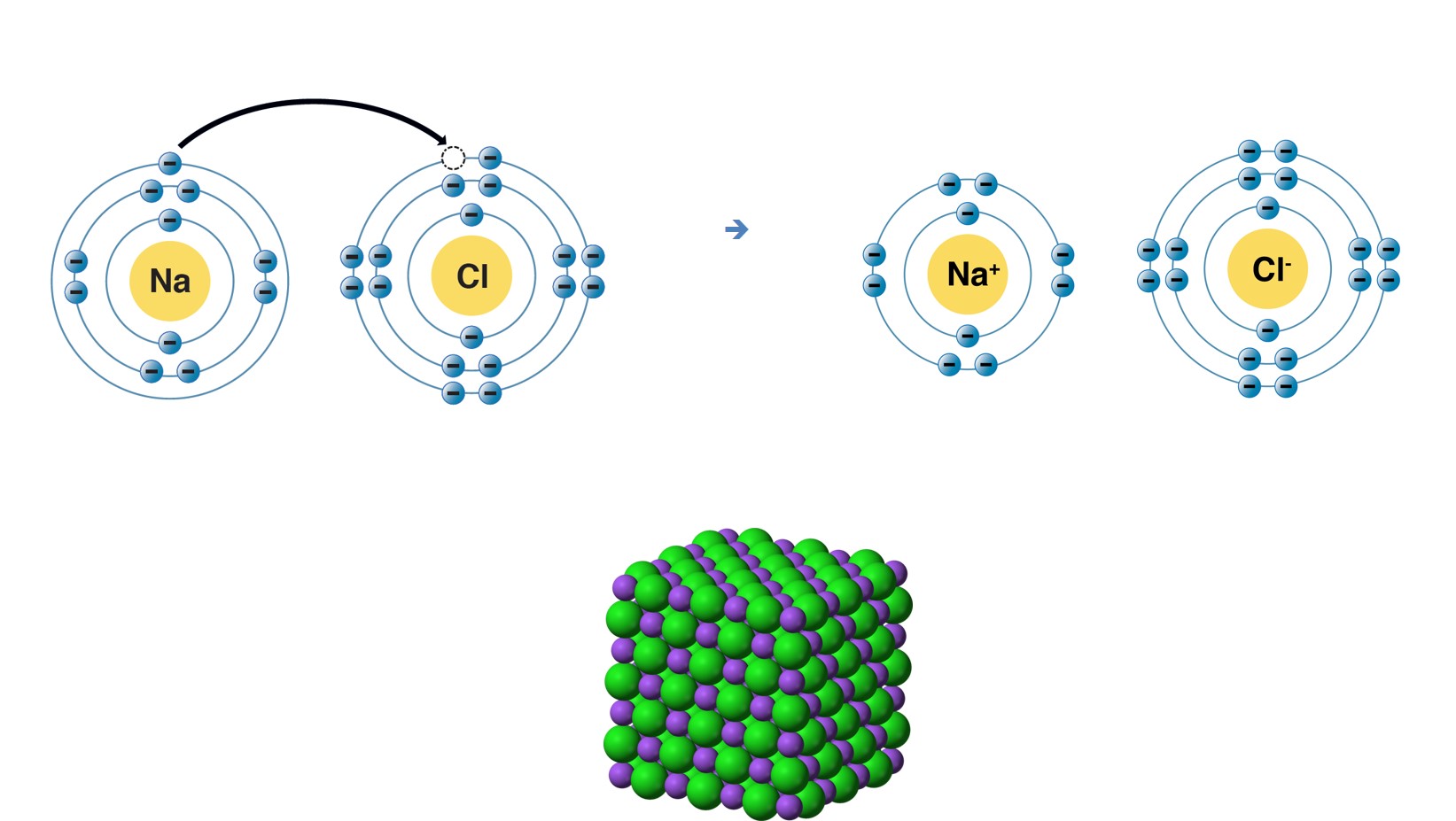
image created by BYU-I student Hannah Crowder Fall 2013
In the image above, the upper left represents the formation of an ionic bond. Sodium gives up one electron and becomes a positively charged sodium ion. In the process, its outer electron shell now has 8 electrons. Chlorine gains one electron and becomes a negatively charged chloride ion with 8 electrons in its outer shell. Upper Right—The negatively charged chloride ions are attracted to the positively charged sodium ions, forming an ionic bond. Bottom—Sodium Chloride crystal. Each sodium ion (purple) is attracted by all of the chloride ions (green) that surround it and each chloride ion is attracted by all of the sodium ions that surround it.
Covalent Bonds
Another way that atoms can fill their outer electron shells is to share electrons. For example, hydrogen has an atomic number of 1 and therefore has 1 electron in its outer electron shell. To fill this shell, hydrogen needs one more electron (recall that the first electron shell will hold a maximum of 2 electrons). One way of filling this shell would be for two hydrogen atoms to unite to form a molecule by sharing electrons with each other. This type of bond, formed by sharing electrons is called a covalent bond. The chemical shorthand for a covalent bond is simply a dash, therefore, the molecule represented in Figure 6 could be expressed as H-H, with the dash representing the shared electrons. Note that the equal sharing of electrons does not cause either atom to have unequal numbers of electrons and protons, hence there is no net – or + charge. Also, unlike ionic bonds, which form crystals, covalent bonds create an intimate relationship between the two atoms, that is, these two atoms are linked directly to each other. You could think of ionic bonds as a group date or hanging out and a covalent bond as marriage. The image below represents a covalent bond between two hydrogen atoms.

image created by MG Fall 2013
Covalent bonds can also be formed by the sharing of more than one pair of electrons. For example, oxygen has an atomic number of 8 with 6 electrons in its outer electron shell. Two oxygen atoms will combine to form oxygen gas, O2, by sharing two pair of electrons, thus completing the outer shell of both oxygen atoms. We refer to this as a double covalent bond and represent it by two dashes, O=O, with each dash again representing a pair of shared electrons. It should be noted that triple covalent bonds are also possible by sharing three pair of electrons, however, in the compounds we will be studying, none have triple covalent bonds.
Depending on the atoms involved in the covalent bonds, the electrons can either be shared equally or the electrons may spend more time with one partner than the other, resulting in unequal sharing of electrons. Two molecules are shown in the figure below. Methane is the image on the left and is a gas that is composed of one carbon and 4 hydrogen atoms. In this molecule the electrons are equally shared between the carbon and each hydrogen forming non-polar covalent bonds. The other image on the right is water. In this molecule, the negatively charged electrons are more strongly attracted to the oxygen and hence spend more time with the oxygen than with the hydrogen. This creates a molecule that has a slight negative charge at one end (the oxygen end) and a slight positive charge at the other end (the hydrogen end). Since the molecule has oppositely charged ends, we refer to this type of bond as a polar covalent bond. Note that the total charge on the molecule is 0, but the ends are charged. Polar covalent bonds and ionic bonds are similar, in that electrons are pulled away from one atom and pushed towards the other. The difference is that with ionic bonds the electrons are completely removed from one atom forming the cation and captured by the other atom forming the anion.
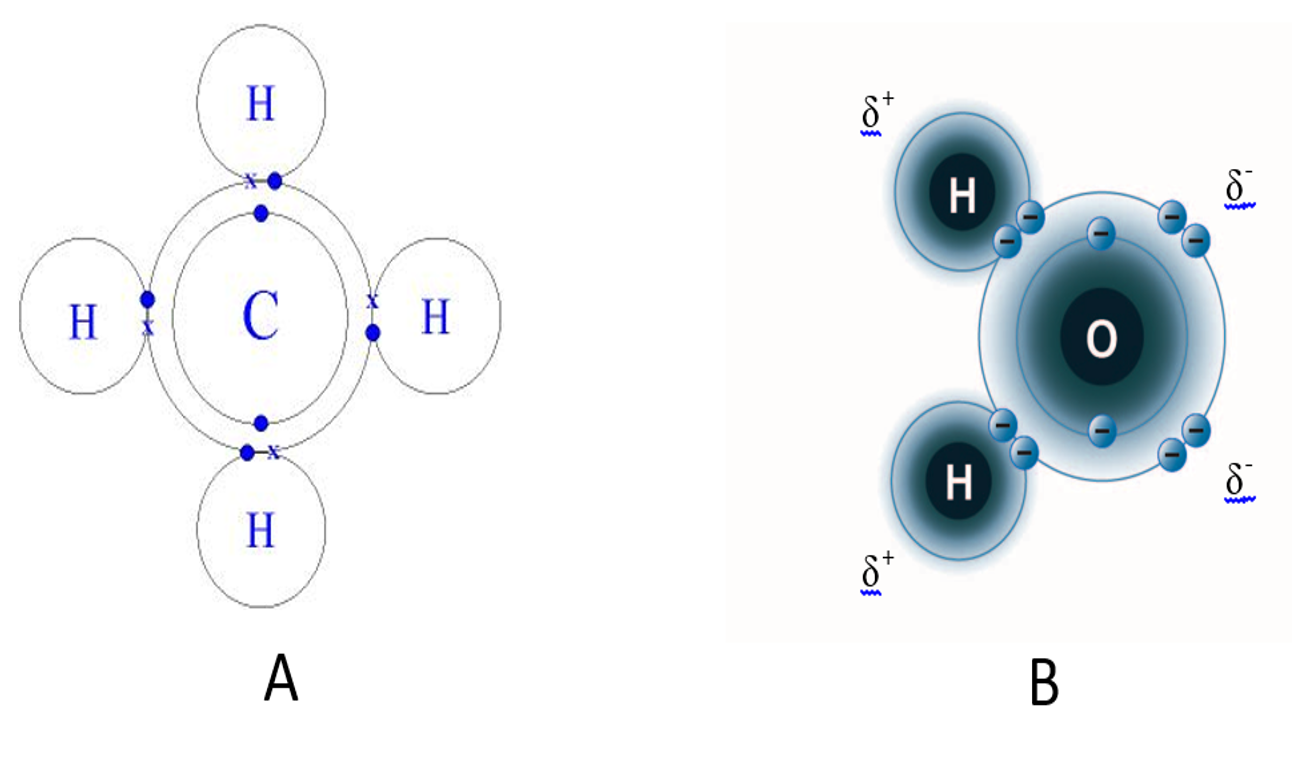
image created by BYU-I student Hannah Crowder Fall 2013
Hydrogen Bonds
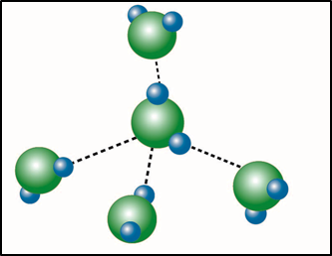
image created by BYU-I student Hannah Crowder Fall 2013
One other interaction of importance in biological systems is called the hydrogen bond. In reality this is not a bond that forms molecules or ionic crystals; rather it is an interaction between molecules containing polar covalent bonds. Because of this, it is referred to as an intermolecular force or an attraction between two molecules. Hydrogen bonds can only occur between molecules containing polar covalent bonds and are a result of attractions between the oppositely charged ends of these molecules. Note that hydrogen bonds and ionic bonds are similar. The difference is that ionic bonds are created by attractions between oppositely charged ions while hydrogen bonds are attractions between oppositely charged ends of polar covalent molecules. Although hydrogen bonds are very weak compared to the other bonds we have discussed, they play important roles in many of the compounds we will be studying in this class. For example, most of the important characteristics of water are due to its ability to form hydrogen bonds with itself and other polar molecules. Likewise, the complex structures of proteins and nucleic acids rely heavily on hydrogen bonding.
**You may use the buttons below to go to the next or previous reading in this Module**


