CELL MEMBRANES-ELECTROPHYSIOLOGY
INTRODUCTION TO ELECTROPHYSIOLOGY
One of the most difficult concepts you will encounter on your journey to becoming a budding student of Anatomy and Physiology is electrophysiology. Electro what? Exactly! However, we are confident that after a few light pages of reading, youll be able to use electrophysiological principles in your everyday conversations. Why is this subject so important? For starters, consider this quote from President John Taylor: "...I could show you upon scientific principles that man himself is a self-registering machine, his eyes, his ears, his nose, the touch, the taste, and all the various senses of the body, are so many media whereby man lays up for himself a record..." (Pres. John Taylor, Journal of Discourses, 26:32.). In more scientific terms, the body is able to take external stimuli (i.e. light, sound, smell, taste) using various sensory organs of the body, and convert these stimuli into unique perceptions in the brain. How the body converts the various types of stimuli from outside sources to inside signals is a major emphasis of electrophysiology. In addition, consider how your brain decides to take your little pinky finger and probe inside your nasal cavity looking for that little gold nugget. How about the magic that occurs when you put your hand in your pocket, and using mechanoreceptors on your fingers, pull out the exact item you seek, without even looking at it! Well, with any luck, after you are done with this section you should be able to explain the "magic" behind these different phenomena.
Ions and Cell Membranes
Review of IonsElectrophysiology, by definition, is the study of the electrical properties of biological cells. It involves measurements of electric current and electrical activity of neurons and other cells. When we talk about electrical properties of cells, what we are really talking about is the cell membrane and how it interacts with ions (charged atoms). You have learned that atoms consist of equal numbers of positively charged protons and negatively charged electrons, unless they give up or accept electrons. Recall that those atoms that give or accept electrons become charged and are called ions. When we take an ionic compound like NaCl and place it into water, the two ions dissociate (come apart) and become free floating ions. In the case of NaCl, these ions are Na+ with a net positive charge and Cl-, with a net negative charge. These ions, Na+ and Cl- along with Ca++, H+, and K+ are very important in the human body.
Separating ChargesWe all know that opposites attract; just walk around the gardens at night on a warm summer evening ... wait, don't do that; at any rate, opposites attract. If we can somehow separate opposite charges from one another and then keep them separated, but maintain the possibility of reuniting, we can generate quite a bit of energy. Consider what happens when you rub a balloon on your head. A good balloon rubbing can elicit hours of FHE fun. When we add work (energy) by rubbing the balloon on the head, we cause a separation of electrical charge. In other words, we cause the electrons from your hair proteins to move from your hair to the balloon surface. The balloon surface gains an attractive force called static electricity. When we put the balloon close to other people's hair, their hair shafts will become attracted to the opposite charges on the balloon. Two important things come out of this analogy; first, opposites attract, and second, it takes energy, or work to separate charges. In the body, the cell membrane has the ability to separate charges by performing work; the charges do not result from the separation of electrons, but rather the separation of charged ions. This separation occurs through the action of an ATP driven pump called the sodium/potassium ATPase pump.
Two very important terms to remember when dealing with the electrical properties of cells are the resting membrane potential and the action potential. These terms will be discussed in more detail through this chapter and it will be important for you to develop a complete and detailed understanding of these concepts.
The word, potential, means having electrical charge. Thus, when we talk about the potential that a cell membrane has because it has separated ions, we refer to it as the resting membrane potential. Later, we will talk about the membrane when it utilizes its potential, and we will call it the action potential. The resting membrane potential is due to specific ions, mainly potassium, and its tendency to diffuse down a concentration gradient until opposed by an electrical force of opposite, but similar magnitude. That is a mouthful!
ANALOGY
To simplify, let's try an analogy. The figure below represents an artificial system of two chambers (A and B) separated by a membrane. We will assume that the membrane is permeable to cations (positively charged ions), but not to anions (negatively charged ions). If we placed two different concentrations of a KCl solution into each chamber, say we placed a 0.1 M solution of KCl in chamber B and a 0.01 M solution of KCl in chamber A, what would happen?
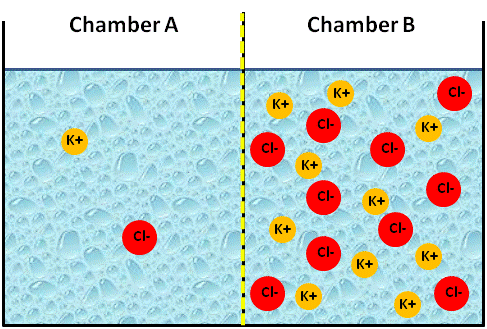
Image Created at BYU-I by JH, 2013.
Artificial container of water with a membrane in the center that is permeable only to cations. The membrane has just been placed in this figure and no ions have moved yet.
Since the membrane is only permeable to cations, K+ will begin to diffuse from chamber B to chamber A, which is less concentrated (see figure below).
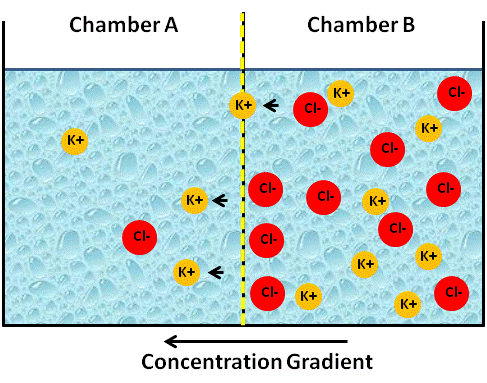
Image Created at BYU-I by JH, 2013.
Artificial container of water with a membrane in the center that is permeable only to cations. Note that the cation K+ is starting to move "down its concentration gradient" to Chamber A.
In biology, we say that K+ moves down its concentration gradient from high to low. When will the diffusion of K+ diffusion stop? You might be tempted to say, the two concentrations are equal, that is, when K+ on side B reaches the same concentration of K+ on side A. However, the definition that equilibrium occurs only when concentrations on either side are the same is only true for non-charged substances. The rules change when things are charged. You see, initially no electrical difference will exist between the two chambers; however, since the membrane is not permeable to Cl-, side B will begin to accumulate negative charges because the positive charges are leaving. Stated another way, chamber A will begin to accumulate positive charges because the K+ cations have moved into this chamber (see figure below).
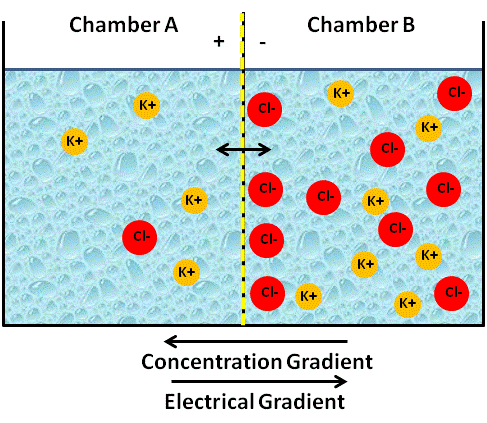
Image Created at BYU-I by JH, 2013.
Artificial container of water with a membrane in the center that is permeable only to cations. Note that the cation K+ moves "down its concentration gradient" to Chamber A, but does not reach a concentration equilibrium because the electrical gradient is "pulling K+ back to Chamber B."
The more K+ that flows from side B to side A, the larger will be the charge difference, and eventually this will result in an electrical force (electrical gradient) that becomes strong enough to oppose the diffusion of K+ across the membrane. The net flow of K+ will stop when the force of the electrical gradient, the attraction from the opposite Cl-charge, equals the force of the concentration gradient. We call this a state of equilibrium, even though the numbers of K+ on side B will still be higher than side A. Because of the opposite force applied by the lonely Cl- ions, the K+ ions will not be able to move to equal concentrations. When the two forces, the electrical gradient and the chemical gradient, equal each other (equilibrium) and the net movement is zero, we call the state resting and can refer to the two combined gradients as the electrochemical gradient. We have now successfully created a state of charge separation and an electrical potential, because side B is now negatively charged compared to side A.
**You may use the buttons below to go to the next or previous reading in this Module**


