METABOLISM
ELECTRON TRANSPORT CHAIN
The Electron Transport Chain is responsible for the synthesis of most of the ATP in our body. In order to understand how the electron transport chain works, it is critical that you have a good understanding of what the mitochondria is and how it is organized. See figure 10 to help you review the structure of this important cell organelle.
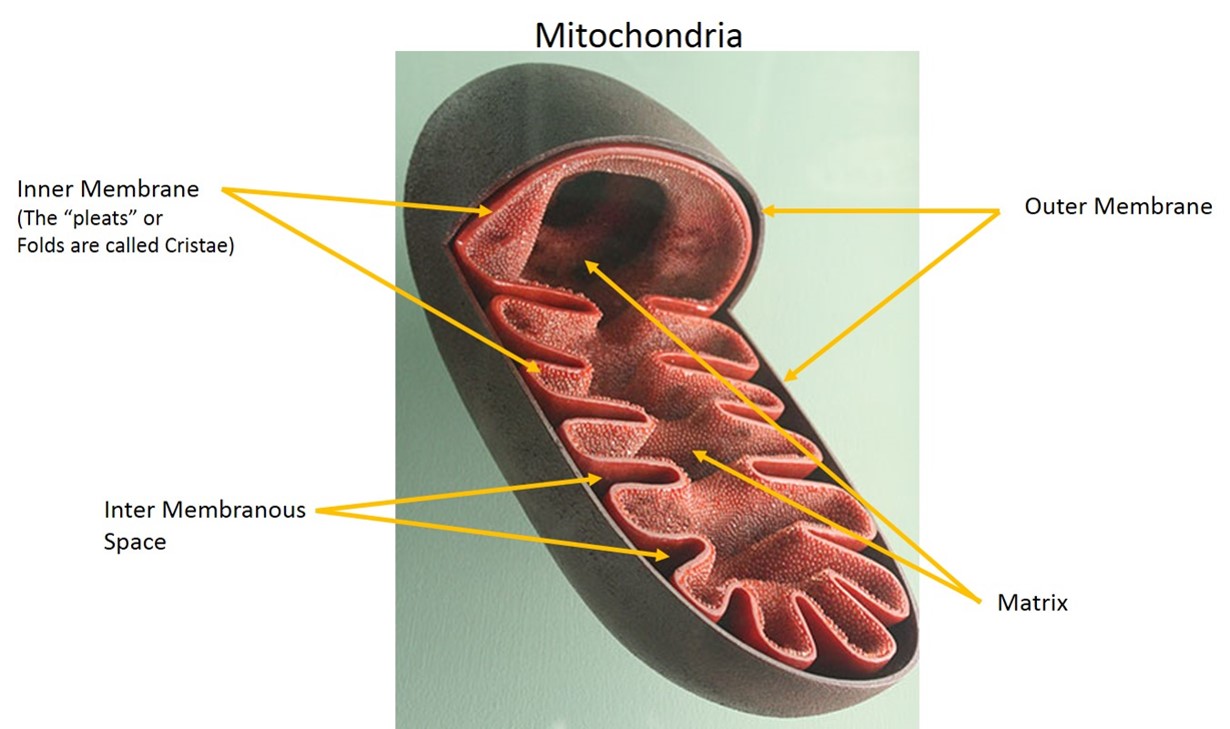
Image derived from File:Überseemuseum Bremen 2009 237.JPG; Author: Sterilgutassistentin; Site: https://commons.wikimedia.org/wiki/File:%C3%9Cberseemuseum_Bremen_2009_237.JPG; License: GNU General Public License as published by the Free Software Foundation; either version 2 of the License, or any later version.
The Mitochondria is a double membrane organelle found within a cell.
The Mitochondria have an inner and an outer membrane. The inner membrane folds in and out on itself and these folds are called Cristae. Cristae increase the total surface area of the inner membrane. The center of the mitochondrion is called the matrix and is analogous to the cytoplasm of a cell. The Electron Transport Chain reactions take place on the inner membrane.
The term, electron transport refers to the proteins on the inner membrane of the mitochondria that will take hydrogen atoms and electrons from NADH and FADH2 and then ultimately use the energy in the electrons to make ATP. Recall that NAD+ and FAD picked up high energy electrons and hydrogens from C-H bonds in glycolysis (from the cytoplasm) and the citric acid cycle (in the matrix of the mitochondria).
In the inner membrane of the mitochondrion is a series of protein complexes that will receive the electrons and pass them from one complex to another (see figure 11). NADH passes 2 high energy electrons onto a protein complex (Complex I) in the inner membrane of the mitochondria. This complex is called NADH dehydrogenase. NADH dehydrogenase does two things. First, it accepts a pair of high energy electrons from NADH second, it uses some of the energy from these electrons to undergo a conformational change. This conformational change is associated with the movement of 4H+ ions from the mitochondrial matrix to the intermembranous space (the space between the inner and outer membranes of the mitochondria). Next, these two new electrons on Complex I are moved to Coenzyme Q (CoQ). Coenzyme Q is also called ubiquinone.
FADH2 also passes a pair of high energy electrons to a protein complex (Complex II), also called Succinate dehydrogenase. Complex II accepts the electrons but does not go through any conformational change that is associated with the movement of H+ ions. However, Complex II does pass the electrons to CoQ just like Complex I did. CoQ is a mobile shuttle that moves easily through the membrane and is able to relocate and react with Complex III. Complex III has a long name (Coenzyme Q-Cytochrome c Oxidoreductase). Complex III also goes by the name Cytochrome bc1 Complex. Complex III will undergo a conformational change that is associated with the movement of 4H+ ions from the mitochondrial matrix to the intermembranous space. The two electrons are then moved from Complex III to Cytochrome C (Cyt c). Cyt c another mobile shuttle that is a soluble protein on in the intermembranous space that moves easily along the membrane and reacts with Complex IV. Complex IV, also called Cytochrome c Oxidase, uses some of the electron energy to undergo a conformational change that is associated with the movement of 2 H+ ions from the mitochondrial matrix to the intermembranous space. Oxygen receives the 2 electrons from Complex IV and reacts with H+ available in the surrounding fluid to make H2O or water.
A review of the figure 11 below reveals that one NADH results in the movement of 10 H+ ions from the mitochondrial matrix to the intermembranous space. One FADH2 results in the active transport of 6 H+ ions. The important message in all of this is that electron energy is used to transport H+ ions to the intermembranous space and this sets up an electrochemical gradient that favors the movement of H+ ions back into the matrix. This is allowed to happen through another protein complex called ATP synthase. The diffusion of H+ ions through ATP synthase is called "chemiosmosis."
ATP synthase is made up of two main components. The component found underneath or in the mitochondrial matrix is capable of rotating and also binds ADP, Pi and ATP. ATP synthase also contain channels that allow the diffusion of H+ ions. As H+ ions diffuse back into the matrix, ATP synthase is physically rotated and enabled to react with ADP and Pi. There is a resulting phosphorylation of ADP and this yields ATP. This process is called Oxidative Phosphorylation.
For each pair of electrons that move from Complex I to Complex IV, about 2.5 ATP can be produced. For each pair of electrons that move from Complex II to Complex IV, about 1.5 ATP can be produced. Therefore, if we round up, it is often stated that each NADH yields 3 ATP while each FADH2 will yield 2 ATP.
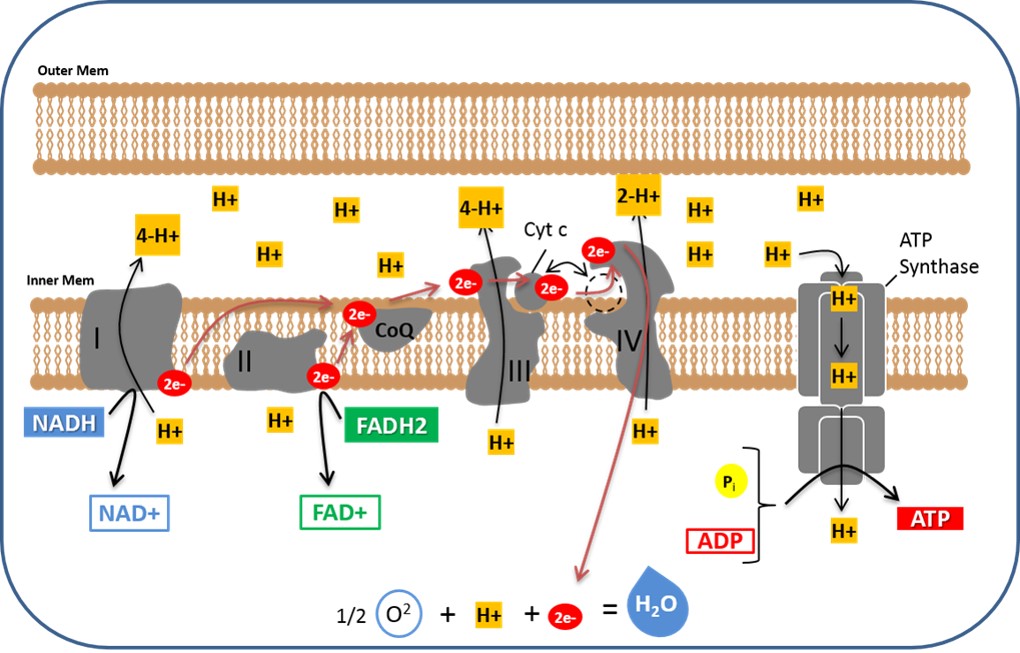
Image created by JS at BYU Idaho F2013.
The image above illustrates the Electron Transport Chain. The protein complexes on the inner mitochondrial membrane use high energy electrons from NADH and FAD2 to move H+ ions to the intermembranous space. The H+ concentration gradient is then used to make ATP through the enzyme complex called ATP Synthase. Oxygen is the final electron acceptor and becomes water.
A quick recap of what has happened so far might go like this: Electrons and hydrogen ions were harvested from the C-H bonds of glucose. These high energy electrons with hydrogen are carried from the reactions of glycolysis and the citric acid cycle to the electron transport chain on the inner membrane of the mitochondria. The electron transport chain takes these high energy electrons and gradually "uses" the energy to pump hydrogen ions to the intermembranous space. As the energy in the electrons is used, the electrons don't have enough energy to form a C-H bond anymore, but they can form an O-H bond. Thus, oxygen comes along and accepts the electrons and hydrogen to form water. The cycle is complete and water can once again be used by a plant somewhere to participate in the photosynthetic reactions that will excite O-H bond electrons again. The hydrogen ions that have been pumped into the intermembranous space are allowed to flow down their electrochemical gradient through ATP synthase. ATP is generated as a result and ATP is used to run the many molecular processes in our cells that keep us healthy and alive. As energy is released in these many reactions of metabolism a little bit is lost as heat. Indeed, metabolism is responsible for a portion of our body heat.
Glucose is not the only molecule with C-H bond energy to use in metabolic reactions. Lipids and Proteins are also metabolized by our cells.
**You may use the buttons below to go to the next or previous reading in this Module**


