CELL MEMBRANES-ELECTROPHYSIOLOGY
MEMBRANE POTENTIALS
In the cell, we use exactly the same principles as the two chamber model, except that we initially use energy, or work to separate the ions from each other (think of our balloon and hair experiment). This energy comes in the form of the Na+/K + ATPase pump. This pump moves three Na+ ions out of the cell and two K+ ions into the cell (see figure below), using ATP (energy) in the process.
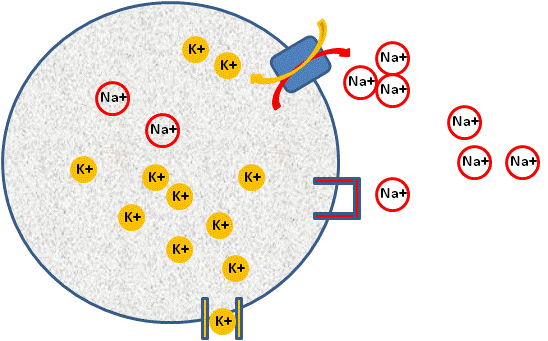
Image Created at BYU-I by JH, 2013.
Diagram of a cell establishing a resting membrane potential. Shown are representations of a K+ channel that allows for the movement of K+ out of the cell, a closed Na+ channel that prevents movement of Na+ into the cell and a Na+/K+ ATPase pump that moves three Na+ ions out of the cell.
This pump is found in almost every cell in the body. The pump creates a concentration gradient for both K+ and Na+. It causes a much higher concentration of K+ to exist inside the cell and a much higher concentration of Na+ to exist outside the cell. This results in a very large concentration gradient for K+ to leave the cell, and a very large concentration gradient for Na+ to come into the cell. However, the cell membrane, under normal conditions will not let Na+ come in, but the membrane is just fine if K+ tries to leave. In more physiological terms, the membrane contains channel proteins for K+ and channel proteins for Na+, however, most channels are closed for Na+ and open for K+, resulting in the membrane being 50-100 times more permeable to K+ than Na+ under resting conditions.
Resting Membrane Potential
Thus, due to the large concentration gradient for K+, it will start to leave the cell by diffusion. As K+ leaves the cell, it will leave behind negative charges, exactly as our model illustrated. In the cell, most of the effect of the lonely negative charge comes from negatively charged proteins. The result is the same; the inside of the cell membrane will start to become negative, with respect to the outside of the membrane. When the membrane reaches a state of equilibrium, we call the state the resting membrane potential and we can actually measure the negative charge inside the cell. We express the membrane charge in millivolts.
Membrane Potentials and Excitable Tissues
So what? Who cares if the inside of the cells are negative? Note the word potential. In this state, the cell now has potential; or the separation of charge. Not every cell has the same potential. All cells have been shown to have a charge difference between the inside and outside of the cell. The size of this charge differs from cell to cell. For example, the resting membrane potential for a neuron is -70mV. Although all tissues exhibit resting membrane potentials, some respond in a unique and predictable way when they are stimulated. We call these cells excitable. Excitable cells have resting potentials that range from -50mV to -85mV, while non-excitable cells have potentials that range from -5 mV to -10 mV. Excitable cells include neurons and skeletal muscle cells, while non-excitable cells include the red blood cell. It doesn't take much imagination to see how neurons and skeletal muscle cells could be much more exciting than red blood cells.
So, how exactly does a membrane potential result in excitability? Before we can fully understand this concept, we need to re-visit the concept of proteins, in particular, those proteins that reside in the cell membrane. First, what are proteins doing in the membrane? Remember that membranes are made up of phospholipids that have hydrophilic ends (love water) and hydrophobic ends (hate water). Because of the nature of phospholipids, the cell membrane is virtually water proof. In fact, it is mostly anything proof, that is, most things cannot cross the barrier made by the phospholipids. However, we have seen that cells have proteins embedded in their membranes that can form gated channels for substances to leave or enter the cells, for instance the K+ channel.
Protein Channels
The K+ channel is an example of a protein that is arranged in the membrane so that it acts as a channel for the passage of K+. Most channel proteins are very specific and will only allow certain things to pass through. At the simplest level, protein channels come in two basic types; those that are open all the time (leak channels), and those that are closed most of the time (gated). Gated channels can be opened by several different types of stimuli. If they open in response to electrical changes, they are called voltage-gated ion channels. Those that open in response to mechanical stimulation are mechanical-gated ion channels and those that open in response to a chemical stimulant are called chemically-gated, or ligand-gated ion channels. K+ can move through the membrane through leak channels or gated channels. Na+ can also move through both types of channels, but in most cells there are small numbers of leak channels for Na+ and large numbers of gated channels for Na+. Thus, under normal resting conditions, Na+ has a very large concentration gradient to enter the cell, but it can't enter because the channel protein for sodium is closed. In fact, for most ions, the case is that their specific protein channel is closed most of the time.
Activation of Voltage Gated Channels
So what determines if a given channel will be closed or open in response to a given stimulus (ie. voltage, mechanical, or ligand)? Proteins have many possible shapes or conformations that are dictated by the way in which their amino acids are arranged. Consider for example the following hypothetical channel.
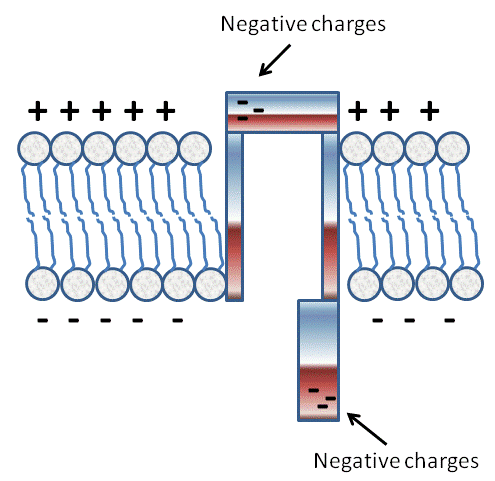
Image Created at BYU-I by JH, 2013.
Depiction of a Voltage Gated Channel. This depiction shows how charges on amino acids can be attracted to or repelled by the "membrane potential" and contribute to the open or closed conformation of the "gates."
Imagine that the protein conformation is such that it acts as a tube through the cell membrane, but it has two doors, a door on the top that opens towards the extracellular compartment and a door on the bottom that opens towards the intracellular compartment.
Now assume that each door portion of the protein has a net negative charge on the tip, opposite the hinge. Under resting conditions the tip of the intracellular door would be repelled by the negative charges, but the tip of the outside door would be attracted by the positive charges. Thus, at rest, the top door would be closed, and the bottom open. If, however, we were able to change the charge, even slightly, on the membrane, then we could affect the conformation of the protein.
If the charge was altered so that the inside suddenly became less negative (see figure below; represented by positive charges) and the outside less positive (see figure below; represent by negative charges), then the doors would flip, the outside would open, and the inside would close, but during the time between doors opening and closing, the channel would be open.
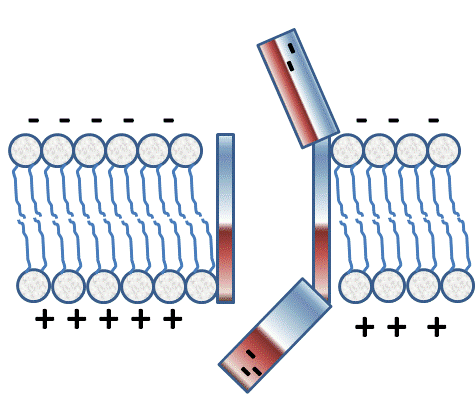
Image Created at BYU-I by JH, 2013.
Depiction of a Voltage Gated channel that has "opened" at the top as the membrane potential switches to be positive on the outside. Notice how the gate on the inside will be facilitated to close.
If the channel were permeable to Na+, then Na+ could move through the channel for a brief moment. Believe it or not, that brief moment of Na+ passing through the membrane is the basis of excitability. Our hypothetical model is an example of a voltage-gated channel. We call it voltage-gated because the gates (doors) of the protein respond to voltage or charge changes (ion movement) in the membrane.
Movement of Ions through Protein Channels
What happens when a channel that was normally closed is opened? When a channel is opened, even though it is for a very brief period of time (less than 0.5msec), it allows for the specific ion to move through the membrane. What happens to the cell during this "open" time depends on the type of ion and the direction of movement. For example, since K+ is high on the inside of the cell, the direction of movement, based on the concentration gradient will be outward or towards the extracellular space. Since K+ is a positively charged ion, moving more K+ out will result in a loss of positive charges; hence the cell will become even more negative. But wasn't K+ already in equilibrium? Why would the movement of K+ change if it was already in equilibrium by simply opening more channels? Well, the equilibrium of K+ was based on the membrane permeability or the number of open channels. By opening more channels, K+ will move until a new equilibrium is established, with respect to the electrochemical gradient. Diffusion will commence until a new electrical gradient is established to oppose the chemical gradient. Thus, the inside of the cell will become more negative because of the increased electrochemical gradient.
Another ion that can cause the membrane potential to become more negative is the Cl- ion. In contrast to K+, the concentration of Cl- is highest on the outside of the cell so that opening of a Cl- channel will allow Cl- to diffuse into the cell, also making the cell more negative. The opposite is true for Na+ and Ca++, both of which are positive ions and have concentrations that are highest on the outside of the cell. Thus, opening channels for either Na+ or Ca++ will result in the inside of the cell becoming more positive as these cations move into the cell. The following table shows the relative amounts of ions (mEq/L) and their distribution as they pertain to the extracellular and intracellular fluids.
|
Ion |
Extracellular Fluid |
Intracellular Fluid |
|---|---|---|
|
Na+ |
142 |
10 |
|
K+ |
4 |
140 |
|
Ca++ |
2.4 |
0.0001 |
|
Mg++ |
1.2 |
58 |
|
Cl- |
103 |
4 |
|
HCO3- |
28 |
10 |
**You may use the buttons below to go to the next or previous reading in this Module**


