Inorganic Chemistry (Atomic Structure)
Matter
Have you ever wondered why water is a liquid, oxygen is a gas, and sugar is a solid? Or why chlorine gas will kill you if you breathe it but when mixed with sodium is necessary for normal body function and we die without it? What about fats? Why won’t they mix with water while salt and sugar do? In the two lessons on basic chemistry we will try to answer these and other questions dealing with the chemicals that make up our bodies.
All living and non-living things are composed of matter. I am sure you learned in high school that matter is anything that occupies space and has mass and that mass is simply the amount of matter that an object contains. Oft times mass and weight are confused. Weight is a function of gravity pulling on matter. For example, your body is composed of a certain quantity of matter, that when acted upon by gravity results in your weight of say 150 pounds. If you took that same amount of matter (mass) to the moon where the gravitational force is only 1/6 of that of the earth you would only weigh 25 pounds (maybe rather than dieting we should all just live on the moon).
Matter is composed of elements. Chemists define elements as substances that cannot be broken down into simpler materials by chemical processes. Some common elements that you have probably heard of are carbon, hydrogen, oxygen, and nitrogen. The building blocks for elements are atoms, which we will discuss in more detail later. In nature there are 92 naturally occurring elements. In addition to these, a number have been artificially produced. Based on their chemical properties, these elements can be organized into what is referred to as the periodic table of the elements. We will refer back to this table frequently as we discuss the basic chemistry of the elements.
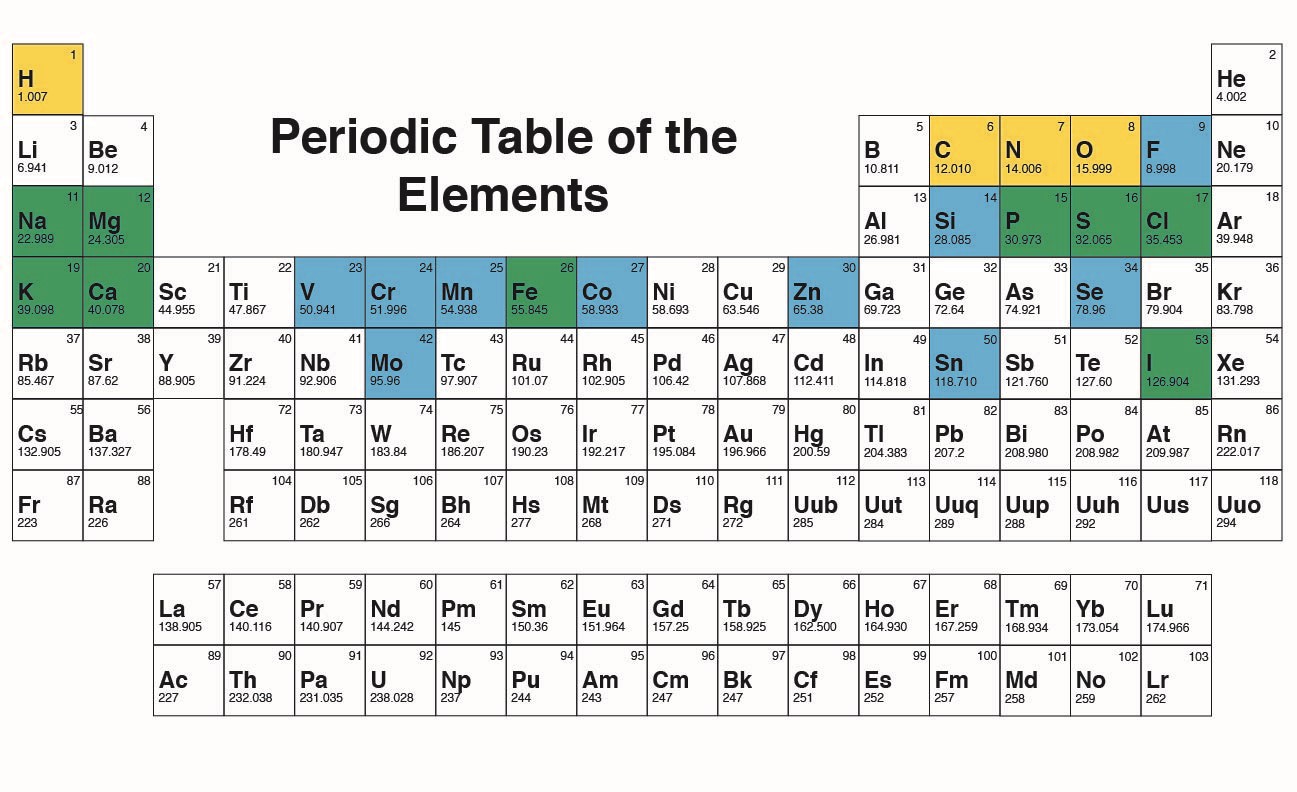
Created by BYU-I student Hannah Crowder Spring of 2013
The figure above is a “Periodic Table of the Elements.” The elements highlighted in yellow make up 96% of our body weight. The 9 elements highlighted in green, along with those in yellow, are considered major essential elements. The elements highlighted in blue are considered minor essential elements and are required only in trace amounts in the body.
Notice that each element is represented by a 1 or 2 letter symbol. Often these symbols are the first letter or letters in the name of the element; H for hydrogen, C for carbon, and He for helium. Occasionally, however, the symbols represent the Latin name for the element, hence the symbol for sodium is Na for the Latin Natrium and the symbol for Potassium is K for the Latin Kalium. Of the 92 naturally occurring elements, 4 make up roughly 96% of our body weight, namely Carbon (C), Hydrogen (H), Oxygen (O) and Nitrogen (N) (Figure above, yellow highlight). In addition to these four, there are a number of other important, but less abundant, elements found in the body. These include phosphorus, sodium, potassium, calcium, magnesium, sulfur, chlorine, iron, and iodine (Figure above, green highlight). Several other elements are also required for normal functioning but only in trace amounts (Figure above, blue highlight).
**You may use the buttons below to go to the next or previous reading in this Module**

